Obesity Represses CYP2R1, the Vitamin D 25-Hydroxylase, in the Liver and Extrahepatic Tissues
- PMID: 33210060
- PMCID: PMC7657391
- DOI: 10.1002/jbm4.10397
Obesity Represses CYP2R1, the Vitamin D 25-Hydroxylase, in the Liver and Extrahepatic Tissues
Abstract
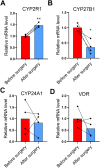
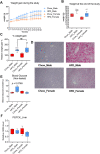
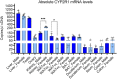
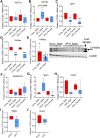
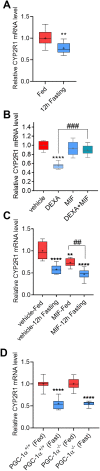
Low plasma level of 25-hydroxyvitamin D (25-OH-D), namely vitamin D deficiency, is associated with obesity and weight loss improves 25-OH-D status. However, the mechanism behind obesity-induced vitamin D deficiency remains unclear. Here, we report that obesity suppresses the expression of cytochrome P450 (CYP) 2R1, the main vitamin D 25-hydroxylase, in both mice and humans. In humans, weight loss induced by gastric bypass surgery increased the expression of CYP2R1 in the s.c. adipose tissue suggesting recovery after the obesity-induced suppression. At the same time, CYP27B1, the vitamin D 1α-hydroxylase, was repressed by the weight loss. In a mouse (C57BL/6N) model of diet-induced obesity, the plasma 25-OH-D was decreased. In accordance, the CYP2R1 expression was strongly repressed in the liver. Moreover, obesity repressed the expression of CYP2R1 in several extrahepatic tissues, the kidney, brown adipose tissue, and testis, but not in the white adipose tissue. Obesity had a similar effect in both male and female mice. In mice, obesity repressed expression of the vitamin D receptor in brown adipose tissue. Obesity also upregulated the expression of the vitamin D receptor and CYP24A1 in the s.c. adipose tissue of a subset of mice; however, no effect was observed in the human s.c. adipose tissue. In summary, we show that obesity affects CYP2R1 expression both in the mouse and human tissues. We suggest that in mouse the CYP2R1 repression in the liver plays an important role in obesity-induced vitamin D deficiency. Currently, it is unclear whether the CYP2R1 downregulation in extrahepatic tissues could contribute to the obesity-induced low plasma 25-OH-D, however, this phenomenon may affect at least the local 25-OH-D concentrations. © 2020 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: 25‐HYDROXYVITAMIN D; CYP24A1; CYP27B1; CYP2R1; HIGH‐FAT DIET; OBESITY; VITAMIN D; VITAMIN D RECEPTOR.
© 2020 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Figures



References
-
- Caprio M, Infante M, Calanchini M, Mammi C, Fabbri A. Vitamin D: not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eating Weight Disord. 2017;22:27–41. - PubMed
-
- Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: an evidence‐based review. J Am Board Fam Med. 2009;22:698–706. - PubMed
-
- Samuel L, Borrell LN. The effect of body mass index on adequacy of serum 25‐hydroxyvitamin D levels in US adults: The National Health and Nutrition Examination Survey 2001 to 2006. Ann Epidemiol. 2014;24:781–4. - PubMed
LinkOut - more resources
Full Text Sources
