Recent Insights on the Role of PPAR-β/δ in Neuroinflammation and Neurodegeneration, and Its Potential Target for Therapy
- PMID: 33210212
- PMCID: PMC7929960
- DOI: 10.1007/s12017-020-08629-9
Recent Insights on the Role of PPAR-β/δ in Neuroinflammation and Neurodegeneration, and Its Potential Target for Therapy
Abstract
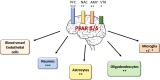
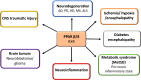
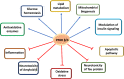
Peroxisome proliferator-activated receptor (PPAR) β/δ belongs to the family of hormone and lipid-activated nuclear receptors, which are involved in metabolism of long-chain fatty acids, cholesterol, and sphingolipids. Similar to PPAR-α and PPAR-γ, PPAR-β/δ also acts as a transcription factor activated by dietary lipids and endogenous ligands, such as long-chain saturated and polyunsaturated fatty acids, and selected lipid metabolic products, such as eicosanoids, leukotrienes, lipoxins, and hydroxyeicosatetraenoic acids. Together with other PPARs, PPAR-β/δ displays transcriptional activity through interaction with retinoid X receptor (RXR). In general, PPARs have been shown to regulate cell differentiation, proliferation, and development and significantly modulate glucose, lipid metabolism, mitochondrial function, and biogenesis. PPAR-β/δ appears to play a special role in inflammatory processes and due to its proangiogenic and anti-/pro-carcinogenic properties, this receptor has been considered as a therapeutic target for treating metabolic syndrome, dyslipidemia, carcinogenesis, and diabetes. Until now, most studies were carried out in the peripheral organs, and despite of its presence in brain cells and in different brain regions, its role in neurodegeneration and neuroinflammation remains poorly understood. This review is intended to describe recent insights on the impact of PPAR-β/δ and its novel agonists on neuroinflammation and neurodegenerative disorders, including Alzheimer's and Parkinson's, Huntington's diseases, multiple sclerosis, stroke, and traumatic injury. An important goal is to obtain new insights to better understand the dietary and pharmacological regulations of PPAR-β/δ and to find promising therapeutic strategies that could mitigate these neurological disorders.
Keywords: Agonists; Alzheimer's disease; Hypoxia/ischemia; Lipid metabolism; Neurodegenerative disorders; Neuroprotection; PPAR delta.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Barroso E, Del Valle J, Porquet D, Vieira Santos AM, Salvadó L, Rodríguez-Rodríguez R, et al. Tau hyperphosphorylation and increased BACE1 and RAGE levels in the cortex of PPARβ/δ-null mice. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2013;1832(8):1241–1248. doi: 10.1016/j.bbadis.2013.03.006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

