Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis
- PMID: 33210341
- PMCID: PMC7791182
- DOI: 10.1111/cpr.12956
Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis
Abstract
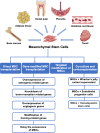
Osteoporosis is a systemic metabolic bone disease with characteristics of bone loss and microstructural degeneration. The personal and societal costs of osteoporosis are increasing year by year as the ageing of population, posing challenges to public health care. Homing disorders, impaired capability of osteogenic differentiation, senescence of mesenchymal stem cells (MSCs), an imbalanced microenvironment, and disordered immunoregulation play important roles during the pathogenesis of osteoporosis. The MSC transplantation promises to increase osteoblast differentiation and block osteoclast activation, and to rebalance bone formation and resorption. Preclinical investigations on MSC transplantation in the osteoporosis treatment provide evidences of enhancing osteogenic differentiation, increasing bone mineral density, and halting the deterioration of osteoporosis. Meanwhile, the latest techniques, such as gene modification, targeted modification and co-transplantation, are promising approaches to enhance the therapeutic effect and efficacy of MSCs. In addition, clinical trials of MSC therapy to treat osteoporosis are underway, which will fill the gap of clinical data. Although MSCs tend to be effective to treat osteoporosis, the urgent issues of safety, transplant efficiency and standardization of the manufacturing process have to be settled. Moreover, a comprehensive evaluation of clinical trials, including safety and efficacy, is still needed as an important basis for clinical translation.
Keywords: clinical trial; mesenchymal stem cells; osteoporosis; preclinical investigation; stem cell therapy.
© 2020 The Authors. Cell Proliferation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Consensus Development Conference . Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646‐650. - PubMed
-
- Singh SV, Tripathi A. An overview of osteoporosis for the practising prosthodontist. Gerodontology. 2010;27(4):308‐314. - PubMed
-
- Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO study group. Osteoporos Int. 1994;4(6):368‐381. - PubMed
-
- Rossini M, Adami S, Bertoldo F, et al. Guidelines for the diagnosis, prevention and management of osteoporosis. Reumatismo. 2016;68(1):1‐39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

