Adsorption of Myoglobin and Corona Formation on Silica Nanoparticles
- PMID: 33210541
- PMCID: PMC7735741
- DOI: 10.1021/acs.langmuir.0c01613
Adsorption of Myoglobin and Corona Formation on Silica Nanoparticles
Abstract
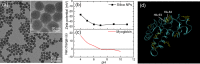
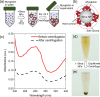
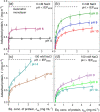
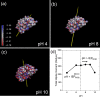
The adsorption of proteins from aqueous medium leads to the formation of protein corona on nanoparticles. The formation of protein corona is governed by a complex interplay of protein-particle and protein-protein interactions, such as electrostatics, van der Waals, hydrophobic, hydrogen bonding, and solvation. The experimental parameters influencing these interactions, and thus governing the protein corona formation on nanoparticles, are currently poorly understood. This lack of understanding is due to the complexity in the surface charge distribution and anisotropic shape of the protein molecules. Here, we investigate the effect of pH and salinity on the characteristics of corona formed by myoglobin on silica nanoparticles. We experimentally measure and theoretically model the adsorption isotherms of myoglobin binding to silica nanoparticles. By combining adsorption studies with surface electrostatic mapping of myoglobin, we demonstrate that a monolayered hard corona is formed in low salinity dispersions, which transforms into a multilayered hard + soft corona upon the addition of salt. We attribute the observed changes in protein adsorption behavior with increasing pH and salinity to the change in electrostatic interactions and surface charge regulation effects. This study provides insights into the mechanism of protein adsorption and corona formation on nanoparticles, which would guide future studies on optimizing nanoparticle design for maximum functional benefits and minimum toxicity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Protein-Nanoparticle Interactions: What Are the Protein-Corona Thickness and Organization?Langmuir. 2019 Aug 20;35(33):10831-10837. doi: 10.1021/acs.langmuir.9b01373. Epub 2019 Aug 6. Langmuir. 2019. PMID: 31333024
-
Nanoparticle-protein complexes mimicking corona formation in ocular environment.Biomaterials. 2016 Dec;109:23-31. doi: 10.1016/j.biomaterials.2016.09.008. Epub 2016 Sep 13. Biomaterials. 2016. PMID: 27648757
-
On the growth of the soft and hard protein corona of mesoporous silica particles with varying morphology.J Colloid Interface Sci. 2022 Apr 15;612:467-478. doi: 10.1016/j.jcis.2021.12.161. Epub 2021 Dec 31. J Colloid Interface Sci. 2022. PMID: 34999551
-
Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review.Int J Biol Macromol. 2021 Feb 1;169:290-301. doi: 10.1016/j.ijbiomac.2020.12.108. Epub 2020 Dec 21. Int J Biol Macromol. 2021. PMID: 33340622 Review.
-
[Progress in the mechanism and influencing factors of the formation of protein corona on nanoparticle surfaces].Sheng Wu Gong Cheng Xue Bao. 2024 May 25;40(5):1448-1468. doi: 10.13345/j.cjb.230835. Sheng Wu Gong Cheng Xue Bao. 2024. PMID: 38783808 Review. Chinese.
Cited by
-
Solution NMR of Nanoparticles in Serum: Protein Competition Influences Binding Thermodynamics and Kinetics.Front Physiol. 2021 Aug 17;12:715419. doi: 10.3389/fphys.2021.715419. eCollection 2021. Front Physiol. 2021. PMID: 34483968 Free PMC article.
-
Deposition of Human-Serum-Albumin-Functionalized Spheroidal Particles on Abiotic Surfaces: Reference Kinetic Results for Bioparticles.Molecules. 2024 Jul 20;29(14):3405. doi: 10.3390/molecules29143405. Molecules. 2024. PMID: 39064983 Free PMC article.
-
Interaction of Colloidal Gold Nanoparticles with Urine and Saliva Biofluids: An Exploratory Study.Nanomaterials (Basel). 2022 Dec 13;12(24):4434. doi: 10.3390/nano12244434. Nanomaterials (Basel). 2022. PMID: 36558287 Free PMC article.
-
Effects of Pore Size and Crosslinking Methods on the Immobilization of Myoglobin in SBA-15.Front Bioeng Biotechnol. 2022 Jan 28;9:827552. doi: 10.3389/fbioe.2021.827552. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35155417 Free PMC article.
-
Mechanism of Myoglobin Molecule Adsorption on Silica: QCM, OWLS and AFM Investigations.Int J Environ Res Public Health. 2021 May 6;18(9):4944. doi: 10.3390/ijerph18094944. Int J Environ Res Public Health. 2021. PMID: 34066515 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

