Pioglitazone for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus
- PMID: 33210751
- PMCID: PMC8092670
- DOI: 10.1002/14651858.CD013516.pub2
Pioglitazone for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus
Abstract
Background: The term prediabetes is used to describe a population with an elevated risk of developing type 2 diabetes mellitus (T2DM). With projections of an increase in the incidence of T2DM, prevention or delay of the disease and its complications is paramount. It is currently unknown whether pioglitazone is beneficial in the treatment of people with increased risk of developing T2DM.
Objectives: To assess the effects of pioglitazone for prevention or delay of T2DM and its associated complications in people at risk of developing T2DM.
Search methods: We searched CENTRAL, MEDLINE, Chinese databases, ICTRP Search Portal and ClinicalTrials.gov. We did not apply any language restrictions. Further, we investigated the reference lists of all included studies and reviews. We tried to contact all study authors. The date of the last search of databases was November 2019 (March 2020 for Chinese databases).
Selection criteria: We included randomised controlled trials (RCTs) with a minimum duration of 24 weeks, and participants diagnosed with intermediate hyperglycaemia with no concomitant diseases, comparing pioglitazone as monotherapy or part of dual therapy with other glucose-lowering drugs, behaviour-changing interventions, placebo or no intervention.
Data collection and analysis: Two review authors independently screened abstracts, read full-text articles and records, assessed risk of bias and extracted data. We performed meta-analyses with a random-effects model and calculated risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, with 95% confidence intervals (CIs) for effect estimates. We evaluated the certainty of the evidence with the GRADE.
Main results: We included 27 studies with a total of 4186 randomised participants. The size of individual studies ranged between 43 and 605 participants and the duration varied between 6 and 36 months. We judged none of the included studies as having low risk of bias across all 'Risk of bias' domains. Most studies identified people at increased risk of T2DM by impaired fasting glucose or impaired glucose tolerance (IGT), or both. Our main outcome measures were all-cause mortality, incidence of T2DM, serious adverse events (SAEs), cardiovascular mortality, nonfatal myocardial infarction or stroke (NMI/S), health-related quality of life (QoL) and socioeconomic effects. The following comparisons mostly reported only a fraction of our main outcome set. Three studies compared pioglitazone with metformin. They did not report all-cause and cardiovascular mortality, NMI/S, QoL or socioeconomic effects. Incidence of T2DM was 9/168 participants in the pioglitazone groups versus 9/163 participants in the metformin groups (RR 0.98, 95% CI 0.40 to 2.38; P = 0.96; 3 studies, 331 participants; low-certainty evidence). No SAEs were reported in two studies (201 participants; low-certainty evidence). One study compared pioglitazone with acarbose. Incidence of T2DM was 1/50 participants in the pioglitazone group versus 2/46 participants in the acarbose group (very low-certainty evidence). No participant experienced a SAE (very low-certainty evidence).One study compared pioglitazone with repaglinide. Incidence of T2DM was 2/48 participants in the pioglitazone group versus 1/48 participants in the repaglinide group (low-certainty evidence). No participant experienced a SAE (low-certainty evidence). One study compared pioglitazone with a personalised diet and exercise consultation. All-cause and cardiovascular mortality, NMI/S, QoL or socioeconomic effects were not reported. Incidence of T2DM was 2/48 participants in the pioglitazone group versus 5/48 participants in the diet and exercise consultation group (low-certainty evidence). No participant experienced a SAE (low-certainty evidence). Six studies compared pioglitazone with placebo. No study reported on QoL or socioeconomic effects. All-cause mortality was 5/577 participants the in the pioglitazone groups versus 2/579 participants in the placebo groups (Peto odds ratio 2.38, 95% CI 0.54 to 10.50; P = 0.25; 4 studies, 1156 participants; very low-certainty evidence). Incidence of T2DM was 80/700 participants in the pioglitazone groups versus 131/695 participants in the placebo groups (RR 0.40, 95% CI 0.17 to 0.95; P = 0.04; 6 studies, 1395 participants; low-certainty evidence). There were 3/93 participants with SAEs in the pioglitazone groups versus 1/94 participants in the placebo groups (RR 3.00, 95% CI 0.32 to 28.22; P = 0.34; 2 studies, 187 participants; very low-certainty evidence). However, the largest study for this comparison did not distinguish between serious and non-serious adverse events. This study reported that 121/303 (39.9%) participants in the pioglitazone group versus 151/299 (50.5%) participants in the placebo group experienced an adverse event (P = 0.03). One study observed cardiovascular mortality in 2/181 participants in the pioglitazone group versus 0/186 participants in the placebo group (RR 5.14, 95% CI 0.25 to 106.28; P = 0.29; very low-certainty evidence). One study observed NMI in 2/303 participants in the pioglitazone group versus 1/299 participants in the placebo group (RR 1.97: 95% CI 0.18 to 21.65; P = 0.58; very low-certainty evidence). Twenty-one studies compared pioglitazone with no intervention. No study reported on cardiovascular mortality, NMI/S, QoL or socioeconomic effects. All-cause mortality was 11/441 participants in the pioglitazone groups versus 12/425 participants in the no-intervention groups (RR 0.85, 95% CI 0.38 to 1.91; P = 0.70; 3 studies, 866 participants; very low-certainty evidence). Incidence of T2DM was 60/1034 participants in the pioglitazone groups versus 197/1019 participants in the no-intervention groups (RR 0.31, 95% CI 0.23 to 0.40; P < 0.001; 16 studies, 2053 participants; moderate-certainty evidence). Studies reported SAEs in 16/610 participants in the pioglitazone groups versus 21/601 participants in the no-intervention groups (RR 0.71, 95% CI 0.38 to 1.32; P = 0.28; 7 studies, 1211 participants; low-certainty evidence). We identified two ongoing studies, comparing pioglitazone with placebo and with other glucose-lowering drugs. These studies, with 2694 participants. may contribute evidence to future updates of this review.
Authors' conclusions: Pioglitazone reduced or delayed the development of T2DM in people at increased risk of T2DM compared with placebo (low-certainty evidence) and compared with no intervention (moderate-certainty evidence). It is unclear whether the effect of pioglitazone is sustained once discontinued. Pioglitazone compared with metformin neither showed advantage nor disadvantage regarding the development of T2DM in people at increased risk (low-certainty evidence). The data and reporting of all-cause mortality, SAEs, micro- and macrovascular complications were generally sparse. None of the included studies reported on QoL or socioeconomic effects.
Trial registration: ClinicalTrials.gov NCT00220961 NCT00352287 NCT00708175 NCT00276497 NCT00994682 NCT00015626 NCT00306826 NCT00470262 NCT00633282 NCT00722631 NCT01006018.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Emil Ørskov Ipsen (EI): had an inadvertent conflict of interest because he had owned a small number of shares with Novo Nordisk A/S before registering the title. Without prompting EI amended the situation by selling the shares on 11 June 2019. The Cochrane Metabolic and Endocrine Disorders group contacted the Cochrane Funding Arbiter for guidance, who agreed to allow the review to proceed with EI as a first author, providing that this issue was clearly explained in the declarations of interest. EI also received a students allowance from the Danish government. The allowance is granted for every full‐time student at universities regardless of field of studies or topic of the master thesis. The review was written as part of his masters' thesis.
Kasper Madsen (KM): none known
Yuan Chi (YC): none known
Ulrik Pedersen‐Bjerregaard (UP): has served on advisory panels for Novo Nordisk and Sanofi Aventis and his institution received an unrestricted research grant from Novo Nordisk.
Bernd Richter (BR): none known
Maria‐Inti Metzendorf (MIM): none known
Bianca Hemmingsen (BH): none known
Figures
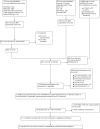

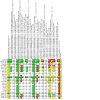












































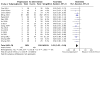






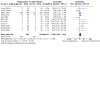

















Comment in
-
Can Pioglitazone Prevent or Delay Type 2 Diabetes in Patients with Prediabetes?Am Fam Physician. 2021 Aug 1;104(2):139-140. Am Fam Physician. 2021. PMID: 34383447 No abstract available.
References
References to studies included in this review
ACT NOW {published data only}
-
- Alonzo R, Chavez-Velazquez A, Martinez R, Velez-Garza J, Carreon M, Musi N, et al. Increase in serum fibroblast growth factor-21 (FGF-21) predicts development of diabetes in IGT subjects and pioglitazone attenuates the increase in FGF-21 and reduces conversion of IGT to T2DM. Diabetes 2016;65:A482. [DOI: 10.2337/db16-1771-2041] - DOI
-
- Anonymous. Pioglitazone reduces the risk of converting impaired glucose tolerance to type 2 diabetes. Australian Journal of Pharmacy 2011;92(1095):82.
Attallah 2007 {published data only}
-
- NCT00352287. Study to determine the effects of human growth hormone and pioglitazone in overweight, prediabetic adults [Effects of GH and pioglitazone in viscerally obese adults with IGT]. clinicaltrials.gov/show/NCT00352287 (first received 14 July 2006).
Bone 2013 {published data only}
-
- Bone HG, Lindsay R, McClung MR, Perez AT, Raanan MG, Spanheimer RG. Effects of pioglitazone on bone in postmenopausal women with impaired fasting glucose or impaired glucose tolerance: a randomized, double-blind, placebo-controlled study. Journal of Clinical Endocrinology and Metabolism 2013;98(12):4691-701. [DOI: 10.1210/jc.2012-4096] - DOI - PubMed
-
- NCT00708175. Efficacy of pioglitazone on bone metabolism in postmenopausal women with impaired fasting glucose [A phase 4, randomized, double-blind, placebo-controlled study to evaluate the effect of pioglitazone compared to placebo on bone metabolism in impaired fasting glucose, postmenopausal women for one year of treatment]. clinicaltrials.gov/show/NCT00708175 (first received 2 July 2008).
Che 2014 {published data only}
-
- Che CJ, Zhao QH. Clinical analysis of pioglitazone dispersible tablets in 38 patients with abnormal glucose tolerance in essential hypertension [吡格列酮分散片对原发性高血压糖耐量异常38例临床分析]. Journal of Medical Theory and Practice [医学理论与实践] 2014;(17):2290-1.
Chen 2007a {published data only}
-
- Chen JH, Wu JN, Su YR, Liu AY, Xu SZ, Huang XY. Intervention study on effects of pioglitazone on patients with impaired glucose tolerance [吡格列酮对糖耐量受损患者的干预治疗研究]. Hainan Medical Journal [海南医学] 2007;18(12):15-7. [DOI: 10.3969/j.issn.1003-6350.2007.12.009] - DOI
Chen 2007b {published data only}
-
- Chen RJ. Effect of pioglitazone on hypertension patients with abnormal glucose tolerance [吡格列酮对高血压合并糖耐量异常患者疗效观察]. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine [深圳中西医结合杂志] 2007;17(6):379-80. [10.3969/j.issn.1007-0893.2007.06.017]
-
- Gong XY. 吡格列酮在高血压病合并糖耐量异常患者中的应用. Chinese Journal of Clinical Medicine [中国现代临床医学] 2008;7(9):9-11.
Deng 2013 {published data only}
-
- Deng CR, Fan HW. Pioglitazone prevents conversion to diabetes in patients with impaired glucose tolerance [吡格列酮预防糖耐量受损患者向糖尿病转化]. Guide of China Medicine [中国医药指南] 2013;11(10):205-7. [DOI: 10.3969/j.issn.1671-8194.2013.10.159] - DOI
Fang 2013 {published data only}
-
- Fang Q, Fang MX, Yao Y, Feng SY, Yang YP, Xue L, et al. Efficacy and safety of pioglitazone for intervention therapy of impaired glucose regulation [吡格列酮干预糖调节受损的疗效和安全性]. Journal of Clinical Research [医学临床研究] 2013;30(2):239-42. [DOI: 10.3969/j.issn.1671-7171.2013.02.012] - DOI
Gao 2011 {published data only}
-
- Gao WX. Curative effect analysis on pioglitazone for treating hypertension with impaired glucose tolerance [吡格列酮对高血压合并糖耐量异常的疗效分析]. Good Health for All [大家健康(中旬版)] 2011;5(5):2-4.
Guo 2009 {published data only}
-
- Guo Y, Li W, Bao H, Fan CH, Zhang XW. Effect of pioglitazone on endothelial function in elderly patients with impaired glucose tolerance [吡格列酮对老年糖耐量低减患者内皮功能的影响]. Chinese Journal of New Drugs [中国新药杂志] 2009;18(24):2323-6.
Guo 2010 {published data only}
-
- Guo Y, Bao H, Li W, Su L, Zhang QW, Miao YD. Effect of pioglitazone on carotid atherosclerosis in old-aged impaired glucose tolerance patients [吡格列酮对老年糖耐量低减患者颈动脉硬化的影响]. Chinese Pharmaceutical Journal [中国药学杂志] 2010;45(10):764-7.
Han 2007 {published data only}
-
- Han SX. Efficacy of pioglitazone in patients with primary hypertension with impaired glucose tolerance [吡格列酮对原发性高血压伴糖耐量减低患者的疗效观察]. Hebei Medical Journal [河北医药] 2007;29(6):595. [DOI: 10.3969/j.issn.1002-7386.2007.06.040] - DOI
IDPP‐2 {published data only}
-
- NCT00276497. Role of pioglitazone in preventing diabetes. clinicaltrials.gov/show/NCT00276497 (first received 13 January 2006).
-
- Ramachandran A, Snehalatha C, Mary S, Selvam S, Kumar CK, Seeli AC, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2). Diabetologia 2009;52(6):1019-26. [DOI: 10.1007/s00125-009-1315-x] - DOI - PubMed
Ke 2006 {published data only}
-
- Ke XH, Tang LH, Tan ML, Huang YZ. Results of intervention of subsidence of glucose tolerance by using rosiglitazone [糖耐量减退吡格列酮干预治疗的研究]. China Tropical Medicine [中国热带医学] 2006;6(9):1622-31. [DOI: 10.3969/j.issn.1009-9727.2006.09.047] - DOI
Li 2017 {published data only}
-
- Li Y. The value of pioglitazone in the treatment of patients with impaired glucose tolerance [吡格列酮在葡萄糖耐量受损患者治疗中应用的价值]. The Journal of Medical Theory and Practice [医学理论与实践] 2017;30(11):1609-11.
Liang 2004 {published data only}
-
- Liang ZQ, Tang L. Observation on the interventive effect of pioglitazone hydrochloride on the IGT population of diabetes mellitus [盐酸吡格列酮干预治疗糖耐量减退的疗效观察]. Journal of Guangxi Medical University [广西医科大学学报] 2004;21(2):165-7. [DOI: 10.3969/j.issn.1005-930X.2004.02.004] - DOI
Shi 2014 {published data only}
-
- Shi J. Intervention of pioglitazone in the development of pre-diabetes [吡格列酮对糖尿病前期病情演变的干预]. Chinese Journal of Trauma and Disability Medicine [中国伤残医学] 2014;22(23):117. [DOI: 10.13214/j.cnki.cjotadm.2014.23.099] - DOI
Tian 2015 {published data only}
-
- Tian F. Effect of pioglitazone on the treatment effect and C-reactive protein of prediabetic patients [探讨吡格列酮对糖尿病前期患者的治疗效果及C反应蛋白的影响]. World Latest Medicine Information [世界最新医学信息文摘] 2015;15(24):131-2. [DOI: 10.3969/j.issn.1671-3141.2015.24.102] - DOI
Wu 2013 {published data only}
-
- Wu YX, Wu TY, Li XJ, Wang W, Zhang Q, Xiong HL, et al. Effects of pioglitazone on ambulatory pulse pressure index in patients with elderly essential hypertension and impaired glucose tolerance [吡格列酮对老年原发性高血压伴糖耐量异常患者动态脉压指数的影响]. Journal of Clinical Cardiology [临床心血管病杂志] 2013;29(6):443-5.
Xiu 2015 {published data only}
-
- Xiu SL, Wang L. Effect of pioglitazone on intervention and C-reactive protein in prediabetic patients [吡格列酮对糖尿病前期患者的干预及C反应蛋白的影响]. Chinese Journal of Medicine [中国医刊] 2015;(1):65-7. [DOI: 10.3969/j.issn.1008-1070.2015.01.021] - DOI
Xu 2011 {published data only}
-
- Xu HS, Lei MX, Kong DM, Xiang H, Xie XY. Intervention of pioglitazone in patients with glucose tolerance disorder [吡格列酮对糖耐量异常患者的干预作用]. Chinese Journal of Gerontology [中国老年学杂志] 2011;31(14):2640-1. [DOI: 10.3969/j.issn.1005-9202.2011.14.013] - DOI
Yi 2015 {published data only}
-
- Yi FS, Li F, Shen LL, Li WJ. Effect of pioglitazone on the treatment effect and C-reactive protein of prediabetic patients [探讨吡格列酮对糖尿病前期患者的治疗效果及C反应蛋白的影响]. Journal of Frontiers of Medicine [医药前沿] 2015;(25):39-40. [DOI: 10.3969/j.issn.2095-1752.2015.25.038] - DOI
Yu 2011 {published data only}
-
- Yu XD, Qian ZM, Ge CL. Effects of pioglitazone on endothelin, C-reactive protein and fibrinogen in patients with impaired glucose tolerance [吡格列酮对糖耐量减低患者内皮素、C反应蛋白、纤维蛋白原的影响]. 中国医师进修杂志 [Chinese Journal of Postgraduates of Medicine] 2011;34(18):62-3.
Zeng 2013 {published data only}
-
- Zeng ZH, Cai F, Qiu JF, Liu YQ, Zhou WZ, Weng LH. The effects of different interventions on impaired glucose regulation [糖调节受损人群不同干预方法的临床效果观察]. Health Research [健康研究] 2013;33(5):370-2, 375. [DOI: 10.3969/j.issn.1674-6449.2013.05.016] - DOI
Zhang 2007 {published data only}
-
- Zhang AH, Li PX, Cao WH, Wei AF, Huang LH. Clinical intervention in patients with IGR [糖调节受损者的临床干预研究]. Acta Academiae Medicinae Weifang [潍坊医学院学报] 2007;29(5):394-7.
Zhang 2015 {published data only}
-
- Zhang FY, Zhao XJ, An YL. Study of pioglitazone intervention effect on impaired glucose regulation [盐酸吡格列酮对葡萄糖调节受损的干预治疗作用]. Journal of Shanxi Medical College for Continuing Education [山西职工医学院学报] 2015;25(1):9-11.
Zhao 2009 {published data only}
-
- Zhao JK. Observation on the effect of pioglitazone intervention in the treatment of glucose tolerance reduction [吡格列酮干预治疗糖耐量减低疗效观察]. 中国实用乡村医生杂志 [Chinese Practical Journal of Rural Doctor] 2009;16(4):32-3. [DOI: 10.3969/j.issn.1672-7185.2009.04.018] - DOI
References to studies excluded from this review
Belfort 2006 {published data only}
-
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. New England Journal of Medicine 2006;355(22):2297-307. - PubMed
ChiCTR‐TRC‐08000099 {published data only}
-
- ChiCTR-TRC-08000099. Multicenter research of early intervention for adult metabolic syndrome in Beijing region. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-08000099 (first received 14 august 2008).
ChiCTR‐TRC‐08000111 {published data only}
-
- ChiCTR-TRC-08000111. A multicenter study of effects of early prevention on adult metabolic syndrome in Beijing region. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-08000111 (first received 14 april 2008).
Cusi 2016 {published data only}
-
- Bril F, Lomonaco R, Orsak B, Finch J, Ortiz-Lopez C, Darland C, et al. Safety of statin therapy in patients with prediabetes or T2DM and NASH: a long-term prospective study. Diabetes 2013;62:A164. [DOI: 10.2337/db13-388-679] - DOI
-
- Bril F, Lomonaco R, Orsak B, Hecht J, Ortiz-Lopez C, Hardies J. Predictors of response after extended pioglitazone treatment in patients with prediabetes or T2DM and nonalcoholic steatohepatitis (NASH). Hepatology (Baltimore, Md.) 2013;58(4 Suppl 1):513A-4A. [DOI: ]
-
- Bril F, Lomonaco R, Orsak B, Hecht J, Tio F, Cusi K. Is treatment of nonalcoholic steatohepatitis with pioglitazone equally effective in patients with prediabetes compared with type 2 diabetes (T2DM)? American Diabetes Association 2017;66:A64.
Durbin 2004 {published data only}
EudraCT2005‐004421‐26 {published data only}
-
- EudraCT2005-004421-26. Effect of pioglitazone on intima media thickness, endothelial function and heart rate variability in patients with impaired glucose tolerance. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:200... (first received 18 July 2006).
EudraCT2006‐002084‐49 {published data only}
-
- EudraCT2006-002084-49. Vascular function in impaired glucose tolerance - effect of pioglitazone. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:200... (first received 13 September 2006).
IRIS 2016 {published data only}
-
- Inzucchi SE, Viscoli CM, Young LH, Furie KL, Gorman M, Lovejoy A, et al. Diabetes prevention with pioglitazone in insulin resistant patients with cerebrovascular disease. American Diabetes Association 2016;Conference: 76th Scientific Sessions of the American Diabetes Association, ADA 2016. United States. 65(Suppl 1):A99.
-
- Mayor S. Pioglitazone may reduce cardiovascular events in high risk patients with prediabetes. BMJ (Clinical Research Ed.) 2016;352:i1029. - PubMed
-
- Spence JD, Viscoli CM, Inzucchi SE, Dearborn-Tomazos J, Ford GA, Gorman M, et al. Correction: pioglitazone therapy in patients with stroke and prediabetes: a post hoc analysis of the IRIS randomized clinical trial (Journal of Physical Chemistry (2019). JAMA Neurology. [DOI: 10.1001/jamaneurol.2019.0475] - DOI - PMC - PubMed
J‐SPIRIT 2015 {published data only}
-
- Tanaka R, Okuma Y, Miyamoto N, Tanaka Y, Yamashiro K, Watada H, et al. Effects of pioglitazone in patients with impaired glucose tolerance after stroke; progress report from J-SPIRIT study. International Journal of Stroke 2010;5 Suppl 2:195.
-
- Tanaka R, Yamashiro K, Okuma Y, Shimura H, Nakamura S, Ueno Y, et al. Effects of pioglitazone for secondary stroke prevention in patients with impaired glucose tolerance and newly diagnosed diabetes: the J-SPIRIT study. Journal of Atherosclerosis and Thrombosis 2015;22(12):1305-16. - PubMed
-
- Tanaka R, Yamashiro K, Tanaka Y, Miyamoto N, Ueno Y, Watanabe M, et al. Effects of pioglitazone in patients with abnormal glucose metabolism after stroke: the J-SPIRIT study. Cerebrovascular Diseases 2013;36 Suppl 2:32 (P59). [DOI: 10.1159/000356197] - DOI
-
- Tanaka R, Yamashiro K, Tanaka Y, Miyamoto N, Ueno Y, Watanabe M, et al. Effects of pioglitazone in patients with abnormal glucose metabolism after stroke. The J-SPIRIT study. Stroke; a Journal of Cerebral Circulation 2013;44(2):Abst.ATP416.
-
- UMINR000015768. Juntendo the stroke prevention study in insulin resistance and impaired glucose tolerance (J-SPIRIT study). upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000015768 (first received 1 December 2005).
Karim 2016 {published data only}
-
- Karim MR, Ahmed H, Paul RK, Chowdhury M, Alam MS, Saha A. Comparative study between pentoxifylline and pioglitazone in the treatment of non-alcoholic fatty liver disease among newly detected glucose intolerant patients. Mymensingh Medical Journal : MMJ 2016;25(2):198-204. - PubMed
Kawamori 1996 {published data only}
-
- Kawamori R, Yoshii H. Drug therapy in subjects with impaired glucose tolerance. Nippon Rinsho - Japanese Journal of Clinical Medicine 1996;54(10):2750-3. - PubMed
Kobayashi 2005 {published data only}
-
- Kobayashi M. Effect of pioglitazone, one of TZDs, on IGT-patients. Nippon Rinsho - Japanese Journal of Clinical Medicine 2005;63 Suppl 2:462-70. - PubMed
Lan 2012 {published data only}
-
- Lan C, Ruan DF. Pioglitazone in the treatment of 60 patients with glucose tolerance reduction [吡格列酮治疗糖耐量减退60例]. China Pharmaceuticals [中国药业] 2012;21(13):80-1. [DOI: 10.3969/j.issn.1006-4931.2012.13.052] - DOI
Li 2009 {published data only}
-
- Li RJ, Li SH, Wu K. Effects of pioglitazone on stent restenosis in coronary heart disease patients with impaired glucose tolerance [吡格列酮对冠心病合并糖耐量异常患者冠脉支架内再狭窄的影响]. Journal of Guangdong Medical College [广东医学院学报] 2009;27(6):620-3. [DOI: 10.3969/j.issn.1005-4057.2009.06.006] - DOI
Liu 2015 {published data only}
-
- Liu MH. The early intervention effect of pioglitazone in IGT patients [吡格列酮对IGT患者早期干预效果的探究]. Guide of China Medicine [中国医药指南] 2015;13(17):158-9.
Nam 2013 {published data only}
-
- Cheon J, Sun Nam J, Kim J, Kim S, Kyung C, Baek H, et al. Additional benefits of pioglitazone to lifestyle modification in patients at increased risk for diabetes and newly developed diabetes. American Diabetes Association 2014;63:A289.
-
- Nam JS, Kim JW, Kang SA, Park JS, Ahn CW, Cha BS, et al. Additional benefits of pioglitazone to lifestyle modification in patients at increased risk for diabetes and newly developed diabetes. American Diabetes Association 2013;62:A596.
NCT00015626 {published data only}
-
- NCT00015626. A clinical trial to prevent the complications of insulin resistance (including type-2 diabetes). ClinicalTrials.gov/show/NCT00015626 (first received 26 april 2001).
NCT00306826 {published data only}
-
- NCT00306826. Pioglitazone in impaired glucose tolerance. clinicaltrials.gov/show/NCT00306826 (first received 24 May 2006).
NCT00470262 {published data only}
-
- NCT00470262. Effects of PPAR ligands on ectopic fat accumulation and inflammation. clinicaltrials.gov/show/NCT00470262 (first received 7 May 2007).
NCT00633282 {published data only}
-
- NCT00633282. Role of pioglitazone and berberine in treatment of non-alcoholic fatty liver disease [Role of pioglitazone and berberine in treatment of non-alcoholic fatty liver disease (NAFLD) patients with impaired glucose regulation or type 2 diabetes mellitus]. clinicaltrials.gov/show/NCT00633282 2008.
NCT00722631 {published data only}
-
- NCT00722631. Anti-inflammatory effects of pioglitazone [Detection of plaque inflammation and visualization of anti-inflammatory effects of pioglitazone on plaque inflammation in subjects with impaired glucose tolerance and type 2 diabetes mellitus by FDG-PET/CT]. clinicaltrials.gov/ct2/show/NCT00722631 2007.
NCT01006018 {published data only}
-
- NCT01006018. DPP-4 inhibition and TZD for DM prevention (DInT DM). clinicaltrials.gov/show/NCT01006018 (first received 7 june 2013).
Norris 2007 {published data only}
UMIN000001035 {published data only}
-
- UMIN000001035. Therapeutic efficacy of pioglitazone for non-alcoholic fatty liver desease(NAFLD) patients with impaired glucose tolerance or type 2 diabetes. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000001215 2008.
Wu 2008 {published data only}
-
- Wu FM. Clinical study of pioglitazone on chronic heart failure and urinary albumin in prediabetic patients [瑞彤对糖尿病前期慢性心衰及尿白蛋白的临床研究]. 实用糖尿病杂志 [Journal of Practical Diabetology] 2008;4(3):46-8. [DOI: 10.3969/j.issn.1673-9191.2008.03.038] - DOI
Yang 2012 {published data only}
-
- Yang HB, Zhao XY, Zhang JY, Du YY, Wang XF. Pioglitazone induces regression and stabilization of coronary atherosclerotic plaques in patients with impaired glucose tolerance. Diabetic Medicine 2012;29(3):359-65. - PubMed
Yokoyama 2007 {published data only}
-
- UMINC000000230. Effects of pioglitazone on coronary atherosclerotic plaques of patients with impaired glucose tolerance or type 2 diabetes mellitus:a randomized,open rabelled,prospective study [Effects of pioglitazone on coronary atherosclerotic plaques:a randomized,open rabelled,prospective study of pioglitazone intervention on coronary atherosclerosis (PIGEON) using intravascular ultrasound]. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000000230 2010.
-
- Yokoyama J, Sutoh N, Higuma T, Horiuchi D, Katoh C, Yokota T, et al. Efficacy and safety of low-dose pioglitazone after primary coronary angioplasty with the use of bare metal stent in patients with acute myocardial infarction and with type 2 diabetes mellitus or impaired glucose tolerance. Heart and Vessels 2007;22(3):146-51. - PubMed
Zhang 2014 {published data only}
-
- Zhang QX. Clinical effect of pioglitazone on hypertension with diabetes mellitus with abnormal glucose tolerance [吡格列酮对高血压合并糖尿病糖耐量异常的临床疗效分析]. Diabetes New World [糖尿病新世界] 2014;34(10):9.
Zhao 2011 {published data only}
-
- Zhao XY, Zhang JY, Yang HB, Li L, Du YY, Wang XF. Pioglitazone regresses coronary plaques in patients of impaired glucose tolerance combined with coronary borderline lesions [吡格列酮缩小糖耐量异常患者冠状动脉斑块面积的临床研究]. Chinese Circulation Journal [中国循环杂志] 2011;26(6):418-21. [DOI: 10.3969/j.issn.1000-3614.2011.06.006] - DOI
Zhou 2006 {published data only}
-
- Zhou WD. The intervention study on impaired glucose tolerance [糖耐量减低的干预研究]. Journal of Clinical Research [医学临床研究] 2006;23(5):714-6.
References to ongoing studies
Beijing prediabetes reversion programme (BPRP) {published data only}
-
- ChiCTR-PRC-06000005. Beijing prediabetes reversion program [Pre-diabetes reversion program in Beijing]. www.chictr.org.cn/showproj.aspx?proj=9520 (first received March 2007).
-
- Duan W, Luo Y, Wang H, Ji L. A compliance evaluation model of lifestyle intervention in prediabetes. American Diabetes Association 2014;63:A208. [DOI: 10.2337/db14-665-832] - DOI
-
- Luo Y, Paul SK, Zhou X, Chang C, Chen W, Guo X, et al. Rationale, design, and baseline characteristics of Beijing Prediabetes Reversion Program: a randomized controlled clinical trial to evaluate the efficacy of lifestyle intervention and/or pioglitazone in reversion to normal glucose tolerance in prediabetes. Journal of Diabetes Research 2017;2017:7602408. [DOI: 10.1155/2017/7602408] - DOI - PMC - PubMed
-
- Luo Y, Zhou X, Wang H, Ji L. Study design and baseline characteristics of Beijing Pre-diabetes Reversion Program (BPRP). American Diabetes Association 2012;61:A626. [DOI: 10.2337/db12-2323-2568] - DOI
NCT02969798 {published data only}
-
- NCT02969798. Pre-diabetes in subject with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) [Preservation of beta cell function in pre-diabetes in subject with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT)]. clinicaltrials.gov/ct2/show/nct02969798 (first received 21 November 2016).
Additional references
Abdul‐Ghani 2006
-
- Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55(5):1430-5. [PMID: ] - PubMed
ADA 1997
-
- American Diabetes Association. Report on the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183-97. - PubMed
ADA 2003
-
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26 Suppl 1:S5-20. - PubMed
ADA 2008
-
- American Diabetes Association. Standards of medical care in diabetes - 2008. Diabetes Care 2008;31 Suppl 1:S12-54. [PMID: ] - PubMed
ADA 2010
ADA 2015
-
- American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care 2015;38 Suppl 1:S1-93. [PMID: ] - PubMed
ADA 2017
-
- American Diabetes Association (ADA). Standards of medical care in diabetes - 2017. Diabetes Care 2017;40 Suppl 1:S 11-33.
Altman 2003
Babai 2018
-
- Babai S, Auclert L, Le-Louet H. Safety data and withdrawal of hepatotoxic drugs. Therapie 2018;S0040-5957(18):30036-2. [DOI: 10.1016/j.therap.2018.02.004] [PMID: ] - PubMed
Bell 2013
-
- Bell ML, McKenzie JE. Designing psycho-oncology randomised trials and cluster randomised trials: variance components and intra-cluster correlation of commonly used psychosocial measures. Psycho-oncology 2013;22:1738-47. - PubMed
Borenstein 2017a
-
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5-18. - PubMed
Borenstein 2017b
-
- Borenstein M. Prediction intervals. www.meta-analysis.com/prediction (accessed 1 September 2019).
Boutron 2014
-
- Boutron I, Altman DG, Hopewell S, Vera-Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32(36):4120-6. - PubMed
Buch 2011
-
- Buch MH, Aletaha D, Emery P, Smolen JS. Reporting of long-term extension studies: lack of consistency calls for consensus. Annals of the Rheumatic Diseases 2011;70(6):886-90. - PubMed
CDC 2015
-
- Centers for Disease Control and Prevention. 2014 National Diabetes Statistics Report. www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html (accessed 15 October 2015).
Cheng 2006
-
- Cheng C, Kushner H, Falkner BE. The utility of fasting glucose for detection of prediabetes. Metabolism: Clinical and Experimental 2006;55(4):434-8. [PMID: ] - PubMed
ClinicalTrials.gov
-
- Search terms: prediabetes drugs. clinicaltrials.gov/ct2/results?term=prediabetes+drugs&Search=Search (accessed 7 June 2019).
Cochrane 2018
-
- Cochrane. CENTRAL creation details. www.cochranelibrary.com/central/central-creation (accessed 21 August 2018).
Cohen 2010
-
- Cohen D. Rosiglitazone: what went wrong? BMJ (Clinical Research Ed.) 2010;341:c4848. [PMID: ] - PubMed
CONSORT 2018
-
- Consolidated Standards of Reporting Trials (CONSORT). The CONSORT statement. www.consort-statement.org (accessed 7 June 2019).
Cooper 2019
Corbett 2014
-
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5(1):79-85. - PubMed
Davies 2019
-
- Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Correction to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2019;62(5):873. [PMID: ] - PubMed
Deeks 2019
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
DeFronzo 1989
-
- DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism: Clinical and Experimental 1989;38(4):387-95. [PMID: ] - PubMed
Diabetes Prevention Program 2002
Diabetes Prevention Program 2009
Diabetes Prevention Program FU 2009
Dunkley 2014
-
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care 2014;37(4):922-33. [PMID: ] - PubMed
Finnish Diabetes Prevention Study Group 2001
-
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343-50. [PMID: ] - PubMed
Gosmanov 2014
-
- Gosmanov AR, Wan J. Low positive predictive value of hemoglobin A1c for diagnosis of prediabetes in clinical practice. American Journal of the Medical Sciences 2014;348(3):191-4. [PMID: ] - PubMed
GRADEproGDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 7 June 2019. Available at gradepro.org.
Hart 2012
Haw 2017
Hemmingsen 2016a
-
- Hemmingsen B, Sonne DP, Metzendorf MI, Richter B. Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD012151. [DOI: 10.1002/14651858.CD012151.pub2] - DOI - PMC - PubMed
Hemmingsen 2016b
-
- Hemmingsen B, Krogh J, Metzendorf MI, Richter B. Sodium-glucose cotransporter (SGLT) 2 inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD012106. [DOI: 10.1002/14651858.CD012106.pub2] - DOI - PMC - PubMed
Hemmingsen 2017
-
- Hemmingsen B, Sonne DP, Metzendorf MI, Richter B. Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012204. [DOI: 10.1002/14651858.CD012204.pub2] - DOI - PMC - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. - PubMed
Higgins 2003
Higgins 2009
Higgins 2019a
-
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hoffmann 2014
-
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. - PubMed
Hoffmann 2017
-
- Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, et al. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ 2017;358:j2998. - PubMed
Hozo 2005
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201-11. - PMC - PubMed
ICH 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice //1997 CFR & ICH Guidelines. In: PA 19063-2043. USA: Barnett International/PAREXEL, 1997.
IDF 2013
-
- International Diabetes Federation (IDF). IDF Diabetes Atlas. 6th edition. Brussels: International Diabetes Federation, 2013.
IEC 2009
Inzucchi 2012
-
- Inzucchi SE. Clinical practice. Diagnosis of diabetes. New England Journal of Medicine 2012;367(6):542-50. [PMID: ] - PubMed
Jones 2015
Kirkham 2010
Liao 2017
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic and meta-analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] - DOI - PMC - PubMed
Lundh 2017
Marshall 2018
Mathieu 2009
-
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977-84. - PubMed
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. Global Evidence Summit, Cape Town, South Africa 13-16 Semptember 2017.
Meader 2014
Megan 2012
-
- Megan B, Pickering RM, Weatherall M. Design, objectives, execution and reporting of published open-label extension studies. Journal of Evaluation in Clinical Practice 2012;18(2):209-15. - PubMed
Merlotti 2014
-
- Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes, Obesity & Metabolism 2014;16(8):719-27. - PubMed
Morris 2013
-
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0-6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 2013;56(7):1489-93. [PMID: ] - PubMed
NDDG 1979
-
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039-57. - PubMed
Ninomiya 2016
-
- Ninomiya H, Hirata A, Kozawa J, Nakata S, Kimura T, Kitamura T, et al. Treatment of mitochondrial diabetes with a peroxisome proliferator-activated receptor (PPAR)-gamma agonist. Internal Medicine (Tokyo, Japan) 2016;55(9):1143-7. [PMID: ] - PubMed
Noel‐Storr 2018
-
- Noel-Storr AH, the Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. Evidence Live 2018, Oxford, UK 18–20 June 2018.
Pang 2018
-
- Pang B, Zhao L-H, Li X-L, Song J, Li Q-W, Liao X, et al. Different intervention strategies for preventing type 2 diabetes mellitus in China: a systematic review and network meta-analysis of randomized controlled trials. Diabetes, Obesity & Metabolism 2018;20(3):718-22. - PubMed
Pi‐Sunyer 2015
-
- Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine 2015;373(1):11-22. - PubMed
Review Manager 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Richter 2006
Richter 2018
Riley 2013
-
- Riley RD, Kauser I, Bland M, Thijs L, Staessen JA, Wang J, et al. Meta-analysis of randomised trials with a continuous outcome according to baseline imbalance and availability of individual participant data. Statistics in Medicine 2013;32(16):2747-66. - PubMed
Schernthaner 2013
Schroll 2015
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors), Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Selvin 2011
Thomas 2017
-
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic review network. Living systematic reviews: 2. combining human and machine effort. Journal of Clinical Epidemiology 2017;S0895-4356(17):30604-2. - PubMed
Viera 2011
-
- Viera AJ. Predisease: when does it make sense? Epidemiologic Reviews 2011;33:122-34. - PubMed
WHO/IDF 2006
-
- World Health Organization/International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20di... 2006;(accessed 17 March 2017).
WHO 1998
-
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15(7):539-53. - PubMed
WHO 1999
-
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. In: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland: World Health Organization, 1999:1-59.
Wood 2008
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

