Neutral Chiral Tetrakis-Iodo-Triazole Halogen-Bond Donor for Chiral Recognition and Enantioselective Catalysis
- PMID: 33210767
- PMCID: PMC7898328
- DOI: 10.1002/chem.202005016
Neutral Chiral Tetrakis-Iodo-Triazole Halogen-Bond Donor for Chiral Recognition and Enantioselective Catalysis
Abstract
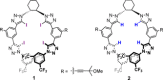
Halogen bonding represents a powerful tool in the field of noncovalent interactions. However, applications in enantioselective recognition and catalysis remain almost nonexistent, due in part to the distinct features of halogen bonds, including long covalent and noncovalent bond distances and high directionality. Herein, this work presents a novel chiral tetrakis-iodo-triazole structure as a neutral halogen bond donor for both chiral anion-recognition and enantioinduction in ion-pair organocatalysis. NMR-titration studies revealed significant differences in anion affinity between the halogen bonding receptor and its hydrogen bonding parent. Selective recognition of chiral dicarboxylates and asymmetric induction in a benchmark organocatalytic reaction were demonstrated using the halogen bond donor. Inversions in the absolute sense of chiral recognition, enantioselectivity, and chiroptical properties relative to the related hydrogen donor were observed. Computational modeling suggested that these effects were the result of distinct anion-binding modes for the halogen- versus hydrogen-bond donors.
Keywords: DFT; NMR titration; chiral anion recognition; halogen bonding; organocatalysis.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Bidentate Chiral Bis(imidazolium)-Based Halogen-Bond Donors: Synthesis and Applications in Enantioselective Recognition and Catalysis.Angew Chem Int Ed Engl. 2020 Apr 20;59(17):6806-6810. doi: 10.1002/anie.201915931. Epub 2020 Mar 18. Angew Chem Int Ed Engl. 2020. PMID: 32045504 Free PMC article.
-
Fine-Tuning Substrate-Catalyst Halogen-Halogen Interactions for Boosting Enantioselectivity in Halogen-Bonding Catalysis.Angew Chem Int Ed Engl. 2023 Aug 28;62(35):e202304781. doi: 10.1002/anie.202304781. Epub 2023 Jul 4. Angew Chem Int Ed Engl. 2023. PMID: 37228095
-
Anion receptors composed of hydrogen- and halogen-bond donor groups: modulating selectivity with combinations of distinct noncovalent interactions.J Am Chem Soc. 2011 Jul 13;133(27):10559-67. doi: 10.1021/ja202096f. Epub 2011 Jun 13. J Am Chem Soc. 2011. PMID: 21667941
-
Frontiers in Halogen and Chalcogen-Bond Donor Organocatalysis.ChemCatChem. 2019 Nov 7;11(21):5198-5211. doi: 10.1002/cctc.201901215. Epub 2019 Aug 30. ChemCatChem. 2019. PMID: 31894187 Free PMC article. Review.
-
Supramolecular Halogen Bonds in Asymmetric Catalysis.Front Chem. 2020 Oct 21;8:599064. doi: 10.3389/fchem.2020.599064. eCollection 2020. Front Chem. 2020. PMID: 33195108 Free PMC article. Review.
Cited by
-
Enhanced enantioselectivity in halogen-bonding catalysis.Commun Chem. 2023 Jul 4;6(1):140. doi: 10.1038/s42004-023-00940-3. Commun Chem. 2023. PMID: 37402818 Free PMC article.
-
Halides as versatile anions in asymmetric anion-binding organocatalysis.Beilstein J Org Chem. 2021 Sep 1;17:2270-2286. doi: 10.3762/bjoc.17.145. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34621390 Free PMC article. Review.
-
Gauging the Strength of the Molecular Halogen Bond via Experimental Electron Density and Spectroscopy.ACS Omega. 2023 Jun 5;8(24):21531-21539. doi: 10.1021/acsomega.3c00619. eCollection 2023 Jun 20. ACS Omega. 2023. PMID: 37360450 Free PMC article.
-
Stereoselective Processes Based on σ-Hole Interactions.Molecules. 2022 Jul 20;27(14):4625. doi: 10.3390/molecules27144625. Molecules. 2022. PMID: 35889497 Free PMC article. Review.
-
N-Heterocyclic Iod(az)olium Salts - Potent Halogen-Bond Donors in Organocatalysis.Chemistry. 2021 Sep 15;27(52):13128-13134. doi: 10.1002/chem.202101961. Epub 2021 Aug 5. Chemistry. 2021. PMID: 34160859 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

