Evaluation of the Accuracy of Current Tubeless Pumps for Continuous Subcutaneous Insulin Infusion
- PMID: 33210949
- PMCID: PMC8080918
- DOI: 10.1089/dia.2020.0525
Evaluation of the Accuracy of Current Tubeless Pumps for Continuous Subcutaneous Insulin Infusion
Erratum in
-
Correction to: Diabetes Technol Ther 2021;23(5):350-357.Diabetes Technol Ther. 2021 Oct;23(10):726. doi: 10.1089/dia.2020.0525.correx. Epub 2021 Sep 15. Diabetes Technol Ther. 2021. PMID: 34525314 Free PMC article. No abstract available.
Abstract
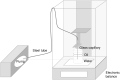
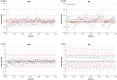
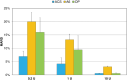
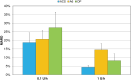
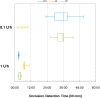
Background: Recently two new tubeless pumps for insulin therapy were introduced. They were tested for accuracy and occlusion detection and compared with the established patch pump Omnipod® (OP). Methods: Using a modified setup for tubeless pumps based on IEC 60601-2-24, the basal rate and bolus delivery of the Accu-Chek® Solo micropump system (ACS) and the A6 TouchCare® System (A6) were measured with a microgravimetric method. Bolus sizes of 0.2, 1, and 10 U, and basal rates of 0.1 and 1 U/h were evaluated in nine repetitions. For each parameter, mean deviation and number of individual boluses or 1-h basal rate windows within ±15% from target were calculated. In addition, occlusion detection time at basal rates of 0.1 and 1 U/h was determined. Results: Mean deviation of boluses of different volumes in the pumps ranged from -3.3% to +4.0% and 40%-100% of individual boluses were within ±15% of the target. During basal rate delivery, 48% to 98% of 1-h windows were within ±15% of the target with a mean deviation between -5.3% and +6.5%. In general, considerable differences between pump models were observed and deviations decreased with increasing doses. In most parameters, ACS was more accurate, and A6 less accurate, than OP. Mean occlusion detection time ranged from ∼3 to 7.5 h at 1 U/h and was >24 h or absent at 0.1 U/h. Conclusions: In this evaluation, significant differences between the tested tubeless pump models were observed that became most evident when regarding delivery errors over short time and small volumes.
Keywords: Accuracy; Continuous subcutaneous insulin infusion; Insulin pump; Occlusion; Patch pump; Tubeless pump.
Conflict of interest statement
R.Z. has received speaker's honoraria and/or served on advisory boards from/of Abbott, Animas, Ascensia, AstraZeneca, Lilly, Novo Nordisk, and Roche Diabetes Care. N.O. has received honoraria for speaking, and research funding for investigator-initiated studies from Dexcom; has received honoraria for participation in advice boards, and research funding from Roche Diabetes Care GmbH. G.F. is general manager of the IDT (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies on the evaluation of BG meters and medical devices for diabetes therapy on its own initiative and on behalf of various companies. G.F. and IDT have received speakers' honoraria or consulting fees from Abbott, Ascensia, Bayer, Dexcom, LifeScan, Menarini Diagnostics, Metronom Health, Novo Nordisk, Roche, Sanofi, Sensile, and Ypsomed. D.W., J.M., and C.H. are employees of the IDT.
Figures


References
-
- American Diabetes Association: Standards of medical care in diabetes-2019. Diabetes Care 2019;24(Suppl1):S1–S193
-
- Adolfsson P, Ziegler R, Hanas R: Continuous subcutaneous insulin infusion: special needs for children. Pediatr Diabetes 2017;18:255–261 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
