Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: A systematic review and meta-analysis
- PMID: 33210980
- PMCID: PMC8250629
- DOI: 10.1177/2050640620972602
Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: A systematic review and meta-analysis
Abstract
Background: The risk of severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) infection and clinical outcomes of coronavirus disease (COVID-19) in inflammatory bowel disease are unclear.
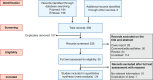
Methods: We searched PubMed and Embase with the keywords: inflammatory bowel disease, Crohn's disease, ulcerative colitis and COVID-19, novel coronavirus and SARS-CoV-2. We included studies reporting the frequency of COVID-19 infection and outcomes (hospitalisation, need for intensive care unit care and mortality) in patients with inflammatory bowel disease. We estimated the pooled incidence of COVID-19 in inflammatory bowel disease and comparative risk vis-a-vis the general population. We also estimated the pooled frequency of outcomes and compared them in patients who received and did not receive drugs for inflammatory bowel disease.
Results: Twenty-four studies were included. The pooled incidence rate of COVID-19 per 1000 patients of inflammatory bowel disease and the general population were 4.02 (95% confidence interval [CI, 1.44-11.17]) and 6.59 [3.25-13.35], respectively, with no increase in relative risk (0.47, 0.18-1.26) in inflammatory bowel disease. The relative risk of the acquisition of COVID-19 was not different between ulcerative colitis and Crohn's disease (1.03, 0.62-1.71). The pooled proportion of COVID-19-positive inflammatory bowel disease patients requiring hospitalisation and intensive care unit care was 27.29% and 5.33% while pooled mortality was 4.27%. The risk of adverse outcomes was higher in ulcerative colitis compared to Crohn's disease. The relative risks of hospitalisation, intensive care unit admission and mortality were lower for patients on biological agents (0.34, 0.19-0.61; 0.49, 0.33-0.72 and 0.22, 0.13-0.38, respectively) but higher with steroids (1.99, 1.64-2.40; 3.41, 2.28-5.11 and 2.70, 1.61-4.55) or 5-aminosalicylate (1.59, 1.39-1.82; 2.38, 1.26-4.48 and 2.62, 1.67-4.11) use.
Conclusion: SARS-CoV-2 infection risk in patients with inflammatory bowel disease is comparable to the general population. Outcomes of COVID-19-positive inflammatory bowel disease patients are worse in ulcerative colitis, those on steroids or 5-aminosalicylates but outcomes are better with biological agents.
Keywords: Crohn's disease; SARS-CoV-2; coronavirus; ulcerative colitis.
© 2020 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC. on behalf of United European Gastroenterology.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures








References
-
- Kirchgesner J, Lemaitre M, Carrat F, et al. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155:337–46. - PubMed
-
- Rubin DT, Abreu MT, Rai V, et al. International Organization for the Study of Inflammatory Bowel Disease. Management of patients with Crohn's disease and ulcerative colitis during the coronavirus disease‐ 2019 pandemic: results of an international meeting. Gastroenterology. 2020;159:6–13. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

