Fetal Growth Restriction: Does an Integrated Maternal Hemodynamic-Placental Model Fit Better?
- PMID: 33211274
- PMCID: PMC8346440
- DOI: 10.1007/s43032-020-00393-2
Fetal Growth Restriction: Does an Integrated Maternal Hemodynamic-Placental Model Fit Better?
Abstract
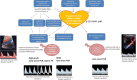
In recent years, a growing interest has arisen regarding the possible relationship between adverse pregnancy outcomes (APOs) and inadequate maternal hemodynamic adaptations to the pregnancy. A possible association between "placental syndromes," such as preeclampsia (PE) and fetal growth restriction (FGR), and subsequent maternal cardiovascular diseases (CVD) later in life has been reported. The two subtypes of FGR show different pathogenetic and clinical features. Defective placentation, due to a poor trophoblastic invasion of the maternal spiral arteries, is believed to play a central role in the pathogenesis of early-onset PE and FGR. Since placental functioning is dependent on the maternal cardiovascular system, a pre-existent or subsequent cardiovascular impairment may play a key role in the pathogenesis of early-onset FGR. Late FGR does not seem to be determined by a primary abnormal placentation in the first trimester. The pathological pathway of late-onset FGR may be due to a primary maternal cardiovascular maladaptation: CV system shows a flat profile and remains similar to those of non-pregnant women. Since the second trimester, when the placenta is already developed and increases its functional request, a hypovolemic state could lead to placental hypoperfusion and to an altered maturation of the placental villous tree and therefore to an altered fetal growth. Thus, this review focalizes on the possible relationship between maternal cardiac function and placentation in the development of both early and late-onset FGR. A better understanding of maternal hemodynamics in pregnancies complicated by FGR could bring various benefits in clinical practice, improving screening and therapeutic tools.
Keywords: Abnormal placentation; Cardiac output; Cardiovascular diseases; Fetal growth restriction; Maternal hemodynamics; Systemic vascular resistance.
© 2020. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99(3):490–496. - PubMed
-
- Gruenwald P. Abnormalities of placental vascularity in relation to intrauterine deprivation and retardation of fetal growth. Significance of avascular chorionic villi. N Y State J Med. 1961;61:1508–1513. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

