Routine laboratory testing to determine if a patient has COVID-19
- PMID: 33211319
- PMCID: PMC8078159
- DOI: 10.1002/14651858.CD013787
Routine laboratory testing to determine if a patient has COVID-19
Abstract
Background: Specific diagnostic tests to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and resulting COVID-19 disease are not always available and take time to obtain results. Routine laboratory markers such as white blood cell count, measures of anticoagulation, C-reactive protein (CRP) and procalcitonin, are used to assess the clinical status of a patient. These laboratory tests may be useful for the triage of people with potential COVID-19 to prioritize them for different levels of treatment, especially in situations where time and resources are limited.
Objectives: To assess the diagnostic accuracy of routine laboratory testing as a triage test to determine if a person has COVID-19.
Search methods: On 4 May 2020 we undertook electronic searches in the Cochrane COVID-19 Study Register and the COVID-19 Living Evidence Database from the University of Bern, which is updated daily with published articles from PubMed and Embase and with preprints from medRxiv and bioRxiv. In addition, we checked repositories of COVID-19 publications. We did not apply any language restrictions.
Selection criteria: We included both case-control designs and consecutive series of patients that assessed the diagnostic accuracy of routine laboratory testing as a triage test to determine if a person has COVID-19. The reference standard could be reverse transcriptase polymerase chain reaction (RT-PCR) alone; RT-PCR plus clinical expertise or and imaging; repeated RT-PCR several days apart or from different samples; WHO and other case definitions; and any other reference standard used by the study authors.
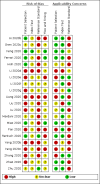
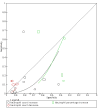
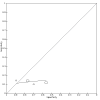
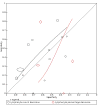
Data collection and analysis: Two review authors independently extracted data from each included study. They also assessed the methodological quality of the studies, using QUADAS-2. We used the 'NLMIXED' procedure in SAS 9.4 for the hierarchical summary receiver operating characteristic (HSROC) meta-analyses of tests for which we included four or more studies. To facilitate interpretation of results, for each meta-analysis we estimated summary sensitivity at the points on the SROC curve that corresponded to the median and interquartile range boundaries of specificities in the included studies.
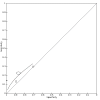
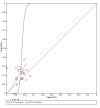
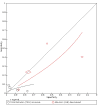
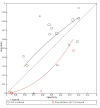
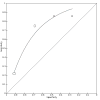
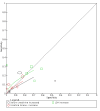
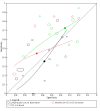
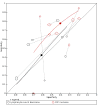
Main results: We included 21 studies in this review, including 14,126 COVID-19 patients and 56,585 non-COVID-19 patients in total. Studies evaluated a total of 67 different laboratory tests. Although we were interested in the diagnotic accuracy of routine tests for COVID-19, the included studies used detection of SARS-CoV-2 infection through RT-PCR as reference standard. There was considerable heterogeneity between tests, threshold values and the settings in which they were applied. For some tests a positive result was defined as a decrease compared to normal vaues, for other tests a positive result was defined as an increase, and for some tests both increase and decrease may have indicated test positivity. None of the studies had either low risk of bias on all domains or low concerns for applicability for all domains. Only three of the tests evaluated had a summary sensitivity and specificity over 50%. These were: increase in interleukin-6, increase in C-reactive protein and lymphocyte count decrease. Blood count Eleven studies evaluated a decrease in white blood cell count, with a median specificity of 93% and a summary sensitivity of 25% (95% CI 8.0% to 27%; very low-certainty evidence). The 15 studies that evaluated an increase in white blood cell count had a lower median specificity and a lower corresponding sensitivity. Four studies evaluated a decrease in neutrophil count. Their median specificity was 93%, corresponding to a summary sensitivity of 10% (95% CI 1.0% to 56%; low-certainty evidence). The 11 studies that evaluated an increase in neutrophil count had a lower median specificity and a lower corresponding sensitivity. The summary sensitivity of an increase in neutrophil percentage (4 studies) was 59% (95% CI 1.0% to 100%) at median specificity (38%; very low-certainty evidence). The summary sensitivity of an increase in monocyte count (4 studies) was 13% (95% CI 6.0% to 26%) at median specificity (73%; very low-certainty evidence). The summary sensitivity of a decrease in lymphocyte count (13 studies) was 64% (95% CI 28% to 89%) at median specificity (53%; low-certainty evidence). Four studies that evaluated a decrease in lymphocyte percentage showed a lower median specificity and lower corresponding sensitivity. The summary sensitivity of a decrease in platelets (4 studies) was 19% (95% CI 10% to 32%) at median specificity (88%; low-certainty evidence). Liver function tests The summary sensitivity of an increase in alanine aminotransferase (9 studies) was 12% (95% CI 3% to 34%) at median specificity (92%; low-certainty evidence). The summary sensitivity of an increase in aspartate aminotransferase (7 studies) was 29% (95% CI 17% to 45%) at median specificity (81%) (low-certainty evidence). The summary sensitivity of a decrease in albumin (4 studies) was 21% (95% CI 3% to 67%) at median specificity (66%; low-certainty evidence). The summary sensitivity of an increase in total bilirubin (4 studies) was 12% (95% CI 3.0% to 34%) at median specificity (92%; very low-certainty evidence). Markers of inflammation The summary sensitivity of an increase in CRP (14 studies) was 66% (95% CI 55% to 75%) at median specificity (44%; very low-certainty evidence). The summary sensitivity of an increase in procalcitonin (6 studies) was 3% (95% CI 1% to 19%) at median specificity (86%; very low-certainty evidence). The summary sensitivity of an increase in IL-6 (four studies) was 73% (95% CI 36% to 93%) at median specificity (58%) (very low-certainty evidence). Other biomarkers The summary sensitivity of an increase in creatine kinase (5 studies) was 11% (95% CI 6% to 19%) at median specificity (94%) (low-certainty evidence). The summary sensitivity of an increase in serum creatinine (four studies) was 7% (95% CI 1% to 37%) at median specificity (91%; low-certainty evidence). The summary sensitivity of an increase in lactate dehydrogenase (4 studies) was 25% (95% CI 15% to 38%) at median specificity (72%; very low-certainty evidence).
Authors' conclusions: Although these tests give an indication about the general health status of patients and some tests may be specific indicators for inflammatory processes, none of the tests we investigated are useful for accurately ruling in or ruling out COVID-19 on their own. Studies were done in specific hospitalized populations, and future studies should consider non-hospital settings to evaluate how these tests would perform in people with milder symptoms.
Copyright © 2020 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Inge Stegeman: has provided freelance consultancy for approved professional organizations and learned societies (physiotherapists, optometrists, opticians), and has no known conflicts of interest in relation to this review.
Eleanor A Ochodo: none known
Fatuma Guleid: none known.
Gea A. Holtman: none known.
Bada Yang: none known.
Jane Cunningham: none known.
Clare Davenport: none known.
Jonathan J Deeks: none known.
Jacqueline Dinnes: none known.
Sabine Dittrich: is employed by FIND. FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know‐how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review.
Devy Emperador: is employed by FIND. FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know‐how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review.
Lotty Hooft: none known.
René Spijker: the Dutch Cochrane Centre (DCC) has received grants for performing commissioned systematic reviews. In no situation, the commissioner had any influence on the results of the work.
Yemisi Takwoingi: none known.
Ann Van den Bruel: none known.
Junfeng Wang: has received consultancy fee from Biomind, an Artificial Intelligence (AI) company providing machine intelligence solutions in medical imaging. The consultancy service was about design of clinical studies, not related to this review. The company had no influence on the results of the work.
Miranda Langendam: none known.
Jan Verbakel: none known.
Mariska MG Leeflang: none known.
Figures




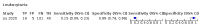


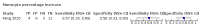

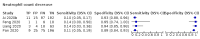
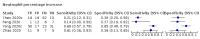
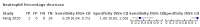
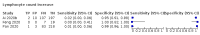

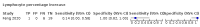
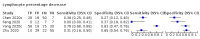
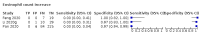
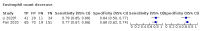
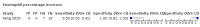
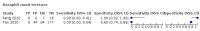
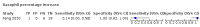
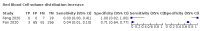
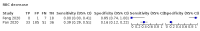

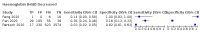
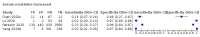
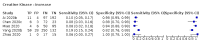
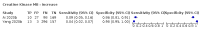
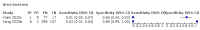

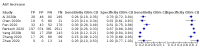



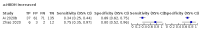
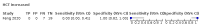

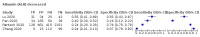
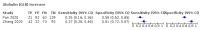

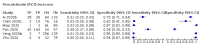
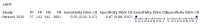
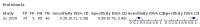
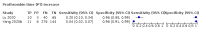
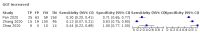
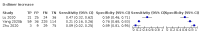
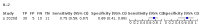
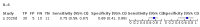
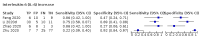
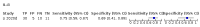
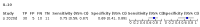
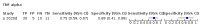
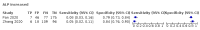

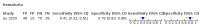
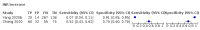
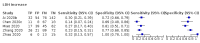
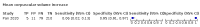
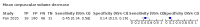

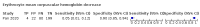

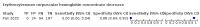
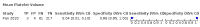
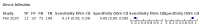
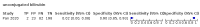
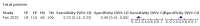

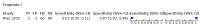
References
References to studies included in this review
Ai 2020b {published data only}
-
- Ai J, Gong J, Xing L, He R, Tian F, Wang J, et al. Analysis of factors associated early diagnosis in coronavirus disease 2019 (COVID-19). medRxiv [Preprint] 2020. [DOI: ]
Chen 2020c {published data only}
Feng 2020 {published data only}
Ferrari 2020 {published data only}
-
- Ferrari D, Motta A, Strollo M, Banfi G, Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clinical Chemistry and Laboratory Medicine 2020;58(7):1095-9. - PubMed
Hsih 2020 {published data only}
Li 2020d {published data only}
Li 2020e {published data only}
Li 2020f {published data only}
-
- Li Q, Ding X, Xia G, Geng Z, Chen F, Wang L, et al. A simple laboratory parameter facilitates early identification of COVID-19 patients. medRxiv [Preprint] 2020. [DOI: ]
Li 2020g {published data only}
-
- Li YX, Wu W, Yang T, Zhou W, Fu YM, Feng QM, et al. Characteristics of peripheral blood leukocyte differential counts in patients with COVID-19. Zhonghua Nei Ke Za Zhi 2020;59(0):E003. - PubMed
Liang 2020 {published data only}
-
- Liang Y, Liang J, Zhou Q, Li X, Lin F, Deng Z, et al. Prevalence and clinical features of 2019 novel coronavirus disease (COVID-19) in the fever clinic of a teaching hospital in Beijing: a single-center, retrospective study. medRxiv [Preprint] 2020. [DOI: ]
Liu 2020 {published data only}
-
- Liu R, Ma Q, Han H, Su H, Liu F, Wu K, et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clinical Chemistry and Laboratory Medicine 2020;58(7):1121-4. - PubMed
Lu 2020 {published data only}
-
- Lu J, Hu S, Fan R, Liu Z, Yin X, Wang Q, et al. ACP risk grade: a simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. medRxiv [Preprint] 2020. [DOI: ]
Mardani 2020 {published data only}
Miao 2020 {published data only}
-
- Miao C, Zhuang J, Jin M, Xiong H, Huang P, Zhao Q, et al. A comparative multi-centre study on the clinical and imaging features of comfirmed and uncomfirmed patients with COVID-19. medRxiv [Preprint] 2020. [DOI: ]
Pan 2020 {published data only}
Rentsch 2020 {published data only}
-
- Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. COVID-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54-75 years. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.09.20059964] - DOI
Yang 2020b {published data only}
-
- Yang L, Bai Y, Qiu Q, Wang T, Jiang L, Liu X, et al. Early-stage vigilance of novel coronavirus pneumonia. Available at ssrn.com/abstract=3544820 [Preprint] 2020. [DOI: ]
Yang 2020c {published data only}
Zhang 2020 {published data only}
-
- Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver International 2020;40(9):2095-2103. - PubMed
Zhao 2020 {published data only}
References to studies excluded from this review
Ai 2020a {published data only}
-
- Ai JW, Zhang HC, Xu T, Wu J, Zhu M, Yu YQ et al. Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi-center study in Eastern China. medRxiv [Preprint] 2020. [DOI: ]
Chen 2020a {published data only}
-
- Chen X, Ling J, Mo P, Zhang Y, Jiang Q, Ma Z, et al. Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients. medRxiv [Preprint] 2020. [DOI: ]
Chen 2020b {published data only}
-
- Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L, et al. The presence of SARS-CoV-2 RNA in feces of COVID-19 patients. Journal of Medical Virology 2020;92(7). [DOI: ] - PubMed
Cheng 2020 {published data only}
-
- Cheng Z, Lu Y, Cao Q, Qin L, Pan Z, Yan F, et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. American Journal of Roentgenology 2020;215(1):121-6. [DOI: ] - PubMed
Giamarellos 2020 {published data only}
Han 2020 {published data only}
-
- Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clinical Chemistry and Laboratory Medicine 2020;58(7). [DOI: ] - PubMed
Kurstjens 2020 {published data only}
-
- Kurstjens S, Horst A, Herpers R, Geerits MW, Kluiters-de Hingh YC, Göttgens EL, et al. Rapid identification of SARS-CoV-2-infected patients at the emergency department using routine testing. medRxiv [Preprint] 2020. [DOI: ] - PubMed
Li 2020a {published data only}
-
- Li J, Li S, Cai Y, Liu Q, Li X, Zeng Z, et al. Epidemiological and clinical characteristics of 17 hospitalized patients with 2019 novel coronavirus Infections outside Wuhan, China. medRxiv [Preprint] 2020. [DOI: ]
Li 2020b {published data only}
-
- Li Y, Wang Z, Hui Y, Tong X, Mao X, Huang L, et al. Clinical characteristics of 77 novel coronavirus 2019 infected patients with respiratory failure in the terminal stage in Wuhan. Available at ssrn.com/abstract=3551325 [Preprint] 2020. [DOI: ]
Li 2020c {published data only}
-
- Li YY, Wang WN, Lei Y, Zhang B, Yang J, Hu JW, et al. Comparison of the clinical characteristics between RNA positive and negative patients clinically diagnosed with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020;43(5):427-430. [DOI: 10.3760/cma.j.cn112147-20200214-00095 ] - PubMed
Ling 2020 {published data only}
Meng 2020 {published data only}
-
- Meng Z, Wang M, Song H, Guo S, Zhou Y, Li W, et al. Development and utilization of an intelligent application for aiding COVID-19 diagnosis. medRxiv [Preprint] 2020. [DOI: ]
Peng 2020 {published data only}
-
- Peng D, Zhang J, Xu Y, Liu Z, Wu P. Clinical analysis and early differential diagnosis of suspected pediatric patients with 2019 novel coronavirus infection. medRxiv [Preprint] 2020. [DOI: ]
Peng 2020a {published data only}
Shi 2020 {published data only}
Song 2020 {published data only}
-
- Song CY, Xu J, He JQ, Lu YQ. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv [Preprint] 2020. [DOI: ]
Spiezia 2020 {published data only}
Sun 2020 {published data only}
Tang 2020 {published data only}
Wang 2020 {published data only}
-
- Wang Z, Weng J, Li Z, Hou R, Zhou L, Ye H, et al. Development and validation of a diagnostic nomogram to predict COVID-19 pneumonia. medRxiv [Preprint] 2020. [DOI: ]
Wu 2020 {published data only}
-
- Wu J, Zhang P, Zhang L, Meng W, Li J, Tong C, et al. Rapid and accurate identification of COVID-19 infection through machine learning based on clinical available blood test results. medRxiv [Preprint] 2020. [DOI: ]
Xu 2020 {published data only}
-
- Xu Y, Li Y, Zeng Q, Lu Z, Li Y, Wu W, et al. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. medRxiv [Preprint] 2020. [DOI: ]
Yang 2020a {published data only}
-
- Yang Y, Shen C, Li J, Yuan J, Yang M, Wang F, et al. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv [Preprint] 2020. [DOI: ]
Additional references
Covidence [Computer program]
-
- Veritas Health Innovation Covidence. Version accessed before 22 October 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2020a
Deeks 2020b
-
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MMG, Spijker R, et al. Diagnosis of SARS-CoV-2 infection and COVID-19: accuracy of signs and symptoms; molecular, antigen, and antibody tests; and routine laboratory markers. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013596. [DOI: 10.1002/14651858.CD013596] - DOI
Dinnes 2020
Macaskill 2010
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from methods.cochrane.org/sdt/handbook-dta-reviews.
McInnes 2020
Review Manager 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager. Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
SAS 2015 [Computer program]
-
- SAS Institute SAS. Version 9.4. Cary, NC, USA: SAS Institute, 2015. Available at www.sas.com.
Schünemann 2020a
-
- Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. [PMID: ] - PubMed
Schünemann 2020b
-
- Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2020;122:142-52. [PMID: ] - PubMed
Struyf 2020
-
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] - DOI - PMC - PubMed
Whiting 2011
-
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al, QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

