The AGCVIII kinase Dw2 modulates cell proliferation, endomembrane trafficking, and MLG/xylan cell wall localization in elongating stem internodes of Sorghum bicolor
- PMID: 33211340
- PMCID: PMC7983884
- DOI: 10.1111/tpj.15086
The AGCVIII kinase Dw2 modulates cell proliferation, endomembrane trafficking, and MLG/xylan cell wall localization in elongating stem internodes of Sorghum bicolor
Abstract
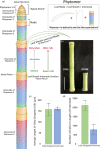
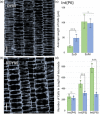
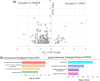
Stems of bioenergy sorghum (Sorghum bicolor L. Moench.), a drought-tolerant C4 grass, contain up to 50 nodes and internodes of varying length that span 4-5 m and account for approximately 84% of harvested biomass. Stem internode growth impacts plant height and biomass accumulation and is regulated by brassinosteroid signaling, auxin transport, and gibberellin biosynthesis. In addition, an AGCVIII kinase (Dw2) regulates sorghum stem internode growth, but the underlying mechanism and signaling network are unknown. Here we provide evidence that mutation of Dw2 reduces cell proliferation in internode intercalary meristems, inhibits endocytosis, and alters the distribution of heteroxylan and mixed linkage glucan in cell walls. Phosphoproteomic analysis showed that Dw2 signaling influences the phosphorylation of proteins involved in lipid signaling (PLDδ), endomembrane trafficking, hormone, light, and receptor signaling, and photosynthesis. Together, our results show that Dw2 modulates endomembrane function and cell division during sorghum internode growth, providing insight into the regulation of monocot stem development.
Keywords: Sorghum bicolor; cell proliferation; cell wall heteroxylan; endocytosis; endomembrane system; mixed linkage glucan; stem growth.
© 2020 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
Federica Brandizzi, PhD, is a senior editor at
Figures





Similar articles
-
Bioenergy sorghum stem growth regulation: intercalary meristem localization, development, and gene regulatory network analysis.Plant J. 2022 Oct;112(2):476-492. doi: 10.1111/tpj.15960. Epub 2022 Sep 22. Plant J. 2022. PMID: 36038985
-
Sorghum Dw2 Encodes a Protein Kinase Regulator of Stem Internode Length.Sci Rep. 2017 Jul 4;7(1):4616. doi: 10.1038/s41598-017-04609-5. Sci Rep. 2017. PMID: 28676627 Free PMC article.
-
High planting density induces the expression of GA3-oxidase in leaves and GA mediated stem elongation in bioenergy sorghum.Sci Rep. 2021 Jan 8;11(1):46. doi: 10.1038/s41598-020-79975-8. Sci Rep. 2021. PMID: 33420129 Free PMC article.
-
Sweet sorghum as a model system for bioenergy crops.Curr Opin Biotechnol. 2012 Jun;23(3):323-9. doi: 10.1016/j.copbio.2011.12.002. Epub 2011 Dec 26. Curr Opin Biotechnol. 2012. PMID: 22204822 Review.
-
Advances in Cell Wall Matrix Research with a Focus on Mixed-Linkage Glucan.Plant Cell Physiol. 2021 Dec 27;62(12):1839-1846. doi: 10.1093/pcp/pcab106. Plant Cell Physiol. 2021. PMID: 34245308 Review.
Cited by
-
Identifying genetic determinants of forage sorghum [Sorghum bicolor (Moench)] adaptation through GWAS.BMC Plant Biol. 2024 Nov 5;24(1):1043. doi: 10.1186/s12870-024-05754-6. BMC Plant Biol. 2024. PMID: 39497045 Free PMC article.
-
A MYB transcription factor underlying plant height in sorghum qHT7.1 and maize Brachytic 1 loci.Plant J. 2024 Dec;120(5):2172-2192. doi: 10.1111/tpj.17111. Epub 2024 Nov 1. Plant J. 2024. PMID: 39485941 Free PMC article.
-
Genetic architecture and molecular regulation of sorghum domestication.aBIOTECH. 2022 Dec 19;4(1):57-71. doi: 10.1007/s42994-022-00089-y. eCollection 2023 Mar. aBIOTECH. 2022. PMID: 37220542 Free PMC article. Review.
-
Deleterious mutations predicted in the sorghum (Sorghum bicolor) Maturity (Ma) and Dwarf (Dw) genes from whole-genome resequencing.Sci Rep. 2023 Oct 3;13(1):16638. doi: 10.1038/s41598-023-42306-8. Sci Rep. 2023. PMID: 37789045 Free PMC article.
-
Bioenergy sorghum stem density increases threefold following internode elongation due to continued accumulation of lignified cell walls and complex regulation of genes involved in cell wall biosynthesis.Biotechnol Biofuels Bioprod. 2025 Jun 4;18(1):58. doi: 10.1186/s13068-025-02659-w. Biotechnol Biofuels Bioprod. 2025. PMID: 40468397 Free PMC article.
References
-
- Ammar, M.‐R. , Humeau, Y. , Hanauer, A. , Nieswandt, B. , Bader, M.‐F. and Vitale, N. (2013) The Coffin‐Lowry Syndrome‐associated protein RSK2 regulates neurite outgrowth through phosphorylation of phospholipase D1 (PLD1) and synthesis of phosphatidic acid. J. Neurosci. 33, 19470–19479. - PMC - PubMed
-
- Barbosa, I.C. and Schwechheimer, C. (2014) Dynamic control of auxin transport‐dependent growth by AGCVIII protein kinases. Curr. Opin. Plant Biol. 22, 108–115. - PubMed
-
- Buschmann, H. , Dols, J. , Kopischke, S. , Peña, E.J. , Andrade‐Navarro, M.A. , Heinlein, M. , Szymanski, D.B. , Zachgo, S. , Doonan, J.H. and Lloyd, C.W. (2015) Arabidopsis KCBP interacts with AIR9 but stays in the cortical division zone throughout mitosis via its MyTH4‐FERM domain. J. Cell Sci. 128, 2033–2046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

