Machine Learning for Electronically Excited States of Molecules
- PMID: 33211478
- PMCID: PMC8391943
- DOI: 10.1021/acs.chemrev.0c00749
Machine Learning for Electronically Excited States of Molecules
Abstract
Electronically excited states of molecules are at the heart of photochemistry, photophysics, as well as photobiology and also play a role in material science. Their theoretical description requires highly accurate quantum chemical calculations, which are computationally expensive. In this review, we focus on not only how machine learning is employed to speed up such excited-state simulations but also how this branch of artificial intelligence can be used to advance this exciting research field in all its aspects. Discussed applications of machine learning for excited states include excited-state dynamics simulations, static calculations of absorption spectra, as well as many others. In order to put these studies into context, we discuss the promises and pitfalls of the involved machine learning techniques. Since the latter are mostly based on quantum chemistry calculations, we also provide a short introduction into excited-state electronic structure methods and approaches for nonadiabatic dynamics simulations and describe tricks and problems when using them in machine learning for excited states of molecules.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



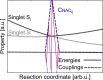
 , shown by their norm) in the adiabatic
basis, in which the triplet state crosses singlet states. (c) The
diagonal, or spin-adiabatic, basis, in which all states are ordered
by their energy and are spin-mixed. Kinetic couplings are shown by
their norm. Note that the ground state is not shown. Potential energy
curves are represented using solid lines and couplings using dashed
lines.
, shown by their norm) in the adiabatic
basis, in which the triplet state crosses singlet states. (c) The
diagonal, or spin-adiabatic, basis, in which all states are ordered
by their energy and are spin-mixed. Kinetic couplings are shown by
their norm. Note that the ground state is not shown. Potential energy
curves are represented using solid lines and couplings using dashed
lines.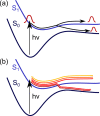

References
-
- Këpuska V.; Bohouta G.. Next-Generation of Virtual Personal Assistants (Microsoft Cortana, Apple Siri, Amazon Alexa and Google Home). IEEE 8th Annual Computing and Communication Workshop and Conference (CCWC), 2018; pp 99–103.
Publication types
LinkOut - more resources
Full Text Sources

