The effects of diet composition and chronic obesity on muscle growth and function
- PMID: 33211595
- PMCID: PMC7944928
- DOI: 10.1152/japplphysiol.00156.2020
The effects of diet composition and chronic obesity on muscle growth and function
Abstract
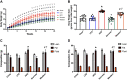
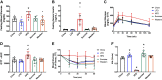
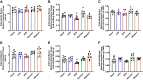
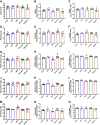
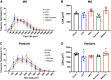
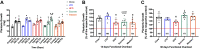
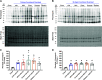
Diet-induced obesity (DIO) is associated with glucose intolerance, insulin resistance (IR), and an increase in intramyocellular lipids (IMCL), which may lead to disturbances in glucose and protein metabolism. To this matter, it has been speculated that chronic obesity and elevated IMCL may contribute to skeletal muscle loss and deficits in muscle function and growth capacity. Thus, we hypothesized that diets with elevated fat content would induce obesity and insulin resistance, leading to a decrease in muscle mass and an attenuated growth response to increased external loading in adult male mice. Male C57BL/6 mice (8 wk of age) were subjected to five different diets, namely, chow, low-dat-diet (LFD), high-fat-diet (HFD), sucrose, or Western diet, for 28 wk. At 25 wk, HFD and Western diets induced a 60.4% and 35.9% increase in body weight, respectively. Interestingly, HFD, but not Western or sucrose, induced glucose intolerance and insulin resistance. Measurement of isometric torque (ankle plantar flexor and ankle dorsiflexor muscles) revealed no effect of DIO on muscle function. At 28 wk of intervention, muscle area and protein synthesis were similar across all diet groups, despite insulin resistance and increased IMCL being observed in HFD and Western diet groups. In response to 30 days of functional overload, an attenuated growth response was observed in only the HFD group. Nevertheless, our results show that DIO alone is not sufficient to induce muscle atrophy and contractile dysfunction in adult male C57BL/6 mice. However, diet composition does have an impact on muscle growth in response to increased external loading.NEW & NOTEWORTHY The effects of diet-induced obesity on skeletal muscle mass are complex and dependent on diet composition and diet duration. The present study results show that chronic exposure to high levels of fatty acids does not affect muscle mass, contractile function, or protein synthesis in obese C57BL/6 mice compared with the consumption of chow. Obesity did result in a delay in load-induced growth; however, only a 45% HFD resulted in attenuated growth following 30 days of functional overload.
Keywords: atrophy; hypertrophy; insulin resistance; intramuscular lipids; muscle function; obesity; skeletal muscle atrophy.
Figures




References
-
- Pahlavani M, Ramalingam L, Miller EK, Scoggin S, Menikdiwela KR, Kalupahana NS, Festuccia WT, Moustaid-Moussa N. Eicosapentaenoic acid reduces adiposity, glucose intolerance and increases oxygen consumption independently of uncoupling protein 1. Mol Nutr Food Res 63: e1800821, 2019. doi: 10.1002/mnfr.201800821. - DOI - PMC - PubMed
-
- Stenholm S, Sallinen J, Koster A, Rantanen T, Sainio P, Heliovaara M, Koskinen S. Association between obesity history and hand grip strength in older adults–exploring the roles of inflammation and insulin resistance as mediating factors. J Gerontol A Biol Sci Med Sci 66A: 341–348, 2011. doi: 10.1093/gerona/glq226. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

