Intravenous sulforhodamine B reduces alveolar surface tension, improves oxygenation, and reduces ventilation injury in a respiratory distress model
- PMID: 33211596
- PMCID: PMC8354824
- DOI: 10.1152/japplphysiol.00421.2020
Intravenous sulforhodamine B reduces alveolar surface tension, improves oxygenation, and reduces ventilation injury in a respiratory distress model
Abstract
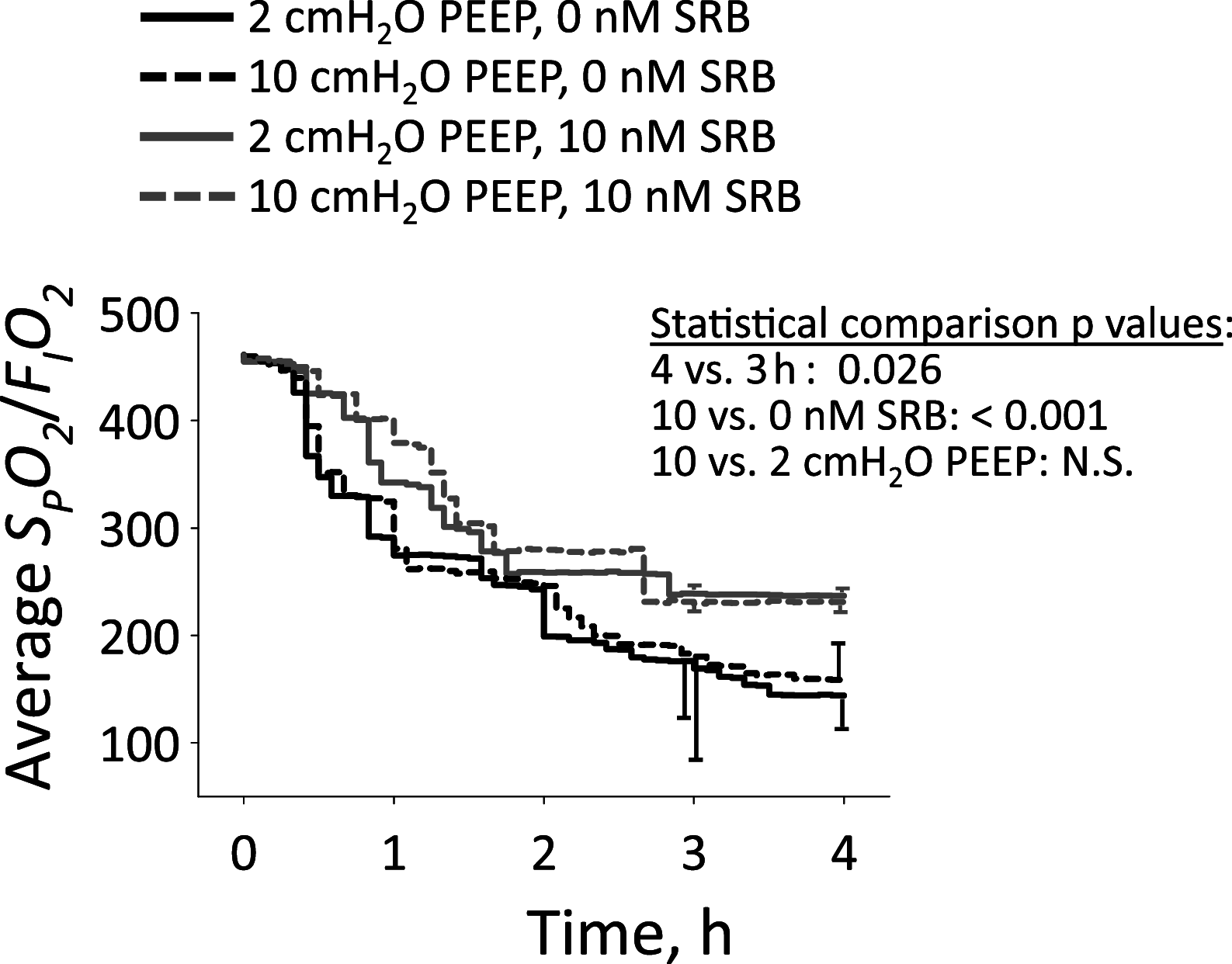
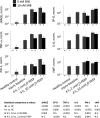
In the neonatal respiratory distress syndrome (NRDS) and acute respiratory distress syndrome (ARDS), mechanical ventilation supports gas exchange but can cause ventilation-induced lung injury (VILI) that contributes to high mortality. Further, surface tension, T, should be elevated and VILI is proportional to T. Surfactant therapy is effective in NRDS but not ARDS. Sulforhodamine B (SRB) is a potential alternative T-lowering therapeutic. In anesthetized male rats, we injure the lungs with 15 min of 42 mL/kg tidal volume, VT, and zero end-expiratory pressure ventilation. Then, over 4 h, we support the rats with protective ventilation-VT of 6 mL/kg with positive end-expiratory pressure. At the start of the support period, we administer intravenous non-T-altering fluorescein (targeting 27 µM in plasma) without or with therapeutic SRB (10 nM). Throughout the support period, we increase inspired oxygen fraction, as necessary, to maintain >90% arterial oxygen saturation. At the end of the support period, we euthanize the rat; sample systemic venous blood for injury marker ELISAs; excise the lungs; combine confocal microscopy and servo-nulling pressure measurement to determine T in situ in the lungs; image fluorescein in alveolar liquid to assess local permeability; and determine lavage protein content and wet-to-dry ratio (W/D) to assess global permeability. Lungs exhibit focal injury. Surface tension is elevated 72% throughout control lungs and in uninjured regions of SRB-treated lungs, but normal in injured regions of treated lungs. SRB administration improves oxygenation, reduces W/D, and reduces plasma injury markers. Intravenous SRB holds promise as a therapy for respiratory distress.NEW & NOTEWORTHY Sulforhodmaine B lowers T in alveolar edema liquid. Given the problematic intratracheal delivery of surfactant therapy for ARDS, intravenous SRB might constitute an alternative therapeutic. In a lung injury model, we find that intravenously administered SRB crosses the injured alveolar-capillary barrier thus reduces T specifically in injured lung regions; improves oxygenation; and reduces the degree of further lung injury. Intravenous SRB administration might help respiratory distress patients, including those with the novel coronavirus, avoid mechanical ventilation or, once ventilated, survive.
Keywords: acute respiratory distress syndrome; neonatal respiratory distress syndrome; oxygenation; surface tension; ventilation-induced lung injury.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Rubaltelli FF, Bonafe L, Tangucci M, Spagnolo A, Dani C. Epidemiology of neonatal acute respiratory disorders. A multicenter study on incidence and fatality rates of neonatal acute respiratory disorders according to gestational age, maternal age, pregnancy complications and type of delivery. Italian Group of Neonatal Pneumology. Biol Neonate 74: 7–15, 1998. doi:10.1159/000014005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

