Sacubitril-valsartan improves conduit vessel function and functional capacity and reduces inflammation in heart failure with reduced ejection fraction
- PMID: 33211601
- PMCID: PMC7944927
- DOI: 10.1152/japplphysiol.00454.2020
Sacubitril-valsartan improves conduit vessel function and functional capacity and reduces inflammation in heart failure with reduced ejection fraction
Abstract
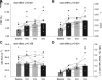
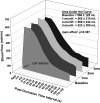
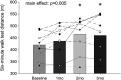
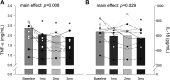
The Prospective comparison of ARNI with angiotensin-converting enzyme inhibitor to Determine Impact on Global Mortality and morbidity in Heart Failure trial identified a marked reduction in the risk of death and hospitalization for heart failure in patients with heart failure with reduced ejection fraction (HFrEF) treated with sacubitril-valsartan (trade name Entresto), but the physiological processes underpinning these improvements are unclear. We tested the hypothesis that treatment with sacubitril-valsartan improves peripheral vascular function, functional capacity, and inflammation in patients with HFrEF. We prospectively studied patients with HFrEF (n = 11, 10 M/1 F, left ventricular ejection fraction = 27 ± 8%) on optimal, guideline-directed medical treatment who were subsequently prescribed sacubitril-valsartan (open-label, uncontrolled, and unblinded). Peripheral vascular function [brachial artery flow-mediated dilation (FMD, conduit vessel function) and reactive hyperemia (RH, microvascular function)], functional capacity [six-minute walk test (6MWT) distance], and the proinflammatory biomarkers tumor necrosis factor-α (TNF-α) and interleukin-18 (IL-18) were obtained at baseline and at 1, 2, and 3 mo of treatment. %FMD improved after 1 mo of treatment, and this favorable response persisted for months 2 and 3 (baseline: 3.25 ± 1.75%; 1 mo: 5.23 ± 2.36%; 2 mo: 5.81 ± 1.79%; 3 mo: 6.35 ± 2.77%), whereas RH remained unchanged. 6MWT distance increased at months 2 and 3 (baseline: 420 ± 92 m; 1 mo: 436 ± 98 m; 2 mo: 465 ± 115 m; 3 mo: 460 ± 110 m), and there was a sustained reduction in TNF-α (baseline: 2.38 ± 1.35 pg/mL; 1 mo: 2.06 ± 1.52 pg/mL; 2 mo: 1.95 ± 1.34 pg/mL; 3 mo: 1.92 ± 1.37 pg/mL) and a reduction in IL-18 at month 3 (baseline: 654 ± 150 pg/mL; 1 mo: 595 ± 140 pg/mL; 2 mo: 601 ± 176 pg/mL; 3 mo: 571 ± 127 pg/mL). This study provides new evidence for the potential of this new drug class to improve conduit vessel function, functional capacity, and inflammation in patients with HFrEF.NEW & NOTEWORTHY We observed an approximately twofold improvement in conduit vessel function (brachial artery FMD), increased functional capacity (6MWT distance), and a reduction in inflammation (TNF-α and IL-18) following 3 mo of sacubitril-valsartan therapy. These findings provide important new information concerning the physiological mechanisms by which this new drug class provokes favorable changes in HFrEF pathophysiology.
Keywords: Entresto; flow-mediated dilation; heart failure; reactive hyperemia.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Sympathoinhibitory effect of sacubitril-valsartan in heart failure with reduced ejection fraction: A pilot study.Auton Neurosci. 2021 Nov;235:102834. doi: 10.1016/j.autneu.2021.102834. Epub 2021 Jun 24. Auton Neurosci. 2021. PMID: 34186274 Free PMC article.
-
Effect of Sacubitril/Valsartan vs Standard Medical Therapies on Plasma NT-proBNP Concentration and Submaximal Exercise Capacity in Patients With Heart Failure and Preserved Ejection Fraction: The PARALLAX Randomized Clinical Trial.JAMA. 2021 Nov 16;326(19):1919-1929. doi: 10.1001/jama.2021.18463. JAMA. 2021. PMID: 34783839 Free PMC article. Clinical Trial.
-
Sacubitril/Valsartan in Patients With Heart Failure and Concomitant End-Stage Kidney Disease.J Am Heart Assoc. 2022 Sep 20;11(18):e026407. doi: 10.1161/JAHA.122.026407. Epub 2022 Sep 5. J Am Heart Assoc. 2022. PMID: 36062622 Free PMC article.
-
Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition.Circulation. 2016 Mar 15;133(11):1115-24. doi: 10.1161/CIRCULATIONAHA.115.018622. Circulation. 2016. PMID: 26976916 Free PMC article. Review.
-
A Review of New Pharmacologic Treatments for Patients With Chronic Heart Failure With Reduced Ejection Fraction.J Clin Pharmacol. 2016 Aug;56(8):936-47. doi: 10.1002/jcph.677. Epub 2015 Dec 30. J Clin Pharmacol. 2016. PMID: 26626162 Review.
Cited by
-
Molecular mechanisms of sacubitril/valsartan in cardiac remodeling.Front Pharmacol. 2022 Aug 8;13:892460. doi: 10.3389/fphar.2022.892460. eCollection 2022. Front Pharmacol. 2022. PMID: 36003518 Free PMC article. Review.
-
When it's time for the sex talk, words matter.Am J Physiol Heart Circ Physiol. 2022 Jan 1;322(1):H66-H70. doi: 10.1152/ajpheart.00556.2021. Epub 2021 Nov 19. Am J Physiol Heart Circ Physiol. 2022. PMID: 34797173 Free PMC article.
-
Effects of sacubitril/valsartan on the functional capacity of real-world patients in Italy: the REAL.IT study on heart failure with reduced ejection fraction.Front Cardiovasc Med. 2024 May 10;11:1347908. doi: 10.3389/fcvm.2024.1347908. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38798920 Free PMC article.
-
Sacubitril/Valsartan and Cognitive Outcomes in Heart Failure With Reduced Ejection Fraction.JACC Adv. 2023 Jun 7;2(4):100372. doi: 10.1016/j.jacadv.2023.100372. eCollection 2023 Jun. JACC Adv. 2023. PMID: 38938237 Free PMC article.
-
Reviewing the Modern Therapeutical Options and the Outcomes of Sacubitril/Valsartan in Heart Failure.Int J Mol Sci. 2022 Sep 26;23(19):11336. doi: 10.3390/ijms231911336. Int J Mol Sci. 2022. PMID: 36232632 Free PMC article. Review.
References
-
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371: 993–1004, 2014. doi:10.1056/NEJMoa1409077. - DOI - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136: e137–e161, 2017. doi:10.1161/CIR.0000000000000509. - DOI - PubMed
-
- Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, Finn PV, Hartley LH, Liu J, Lefkowitz M, Shi V, Zile MR, Solomon SD. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J 36: 1990–1997, 2015. doi:10.1093/eurheartj/ehv186. - DOI - PubMed
-
- Bunsawat K, Ratchford SM, Alpenglow JK, Park SH, Jarrett CL, Stehlik J, Drakos SG, Richardson RS, Wray DW. Chronic antioxidant administration restores macrovascular function in patients with heart failure with reduced ejection fraction. Exp Physiol 105: 1384–1395, 2020. doi:10.1113/EP088686. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

