The risk of Plasmodium vivax parasitaemia after P. falciparum malaria: An individual patient data meta-analysis from the WorldWide Antimalarial Resistance Network
- PMID: 33211712
- PMCID: PMC7676739
- DOI: 10.1371/journal.pmed.1003393
The risk of Plasmodium vivax parasitaemia after P. falciparum malaria: An individual patient data meta-analysis from the WorldWide Antimalarial Resistance Network
Abstract
Background: There is a high risk of Plasmodium vivax parasitaemia following treatment of falciparum malaria. Our study aimed to quantify this risk and the associated determinants using an individual patient data meta-analysis in order to identify populations in which a policy of universal radical cure, combining artemisinin-based combination therapy (ACT) with a hypnozoitocidal antimalarial drug, would be beneficial.
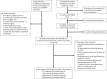
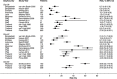
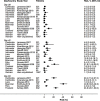
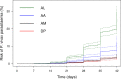
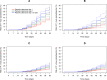
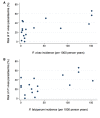
Methods and findings: A systematic review of Medline, Embase, Web of Science, and the Cochrane Database of Systematic Reviews identified efficacy studies of uncomplicated falciparum malaria treated with ACT that were undertaken in regions coendemic for P. vivax between 1 January 1960 and 5 January 2018. Data from eligible studies were pooled using standardised methodology. The risk of P. vivax parasitaemia at days 42 and 63 and associated risk factors were investigated by multivariable Cox regression analyses. Study quality was assessed using a tool developed by the Joanna Briggs Institute. The study was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42018097400). In total, 42 studies enrolling 15,341 patients were included in the analysis, including 30 randomised controlled trials and 12 cohort studies. Overall, 14,146 (92.2%) patients had P. falciparum monoinfection and 1,195 (7.8%) mixed infection with P. falciparum and P. vivax. The median age was 17.0 years (interquartile range [IQR] = 9.0-29.0 years; range = 0-80 years), with 1,584 (10.3%) patients younger than 5 years. 2,711 (17.7%) patients were treated with artemether-lumefantrine (AL, 13 studies), 651 (4.2%) with artesunate-amodiaquine (AA, 6 studies), 7,340 (47.8%) with artesunate-mefloquine (AM, 25 studies), and 4,639 (30.2%) with dihydroartemisinin-piperaquine (DP, 16 studies). 14,537 patients (94.8%) were enrolled from the Asia-Pacific region, 684 (4.5%) from the Americas, and 120 (0.8%) from Africa. At day 42, the cumulative risk of vivax parasitaemia following treatment of P. falciparum was 31.1% (95% CI 28.9-33.4) after AL, 14.1% (95% CI 10.8-18.3) after AA, 7.4% (95% CI 6.7-8.1) after AM, and 4.5% (95% CI 3.9-5.3) after DP. By day 63, the risks had risen to 39.9% (95% CI 36.6-43.3), 42.4% (95% CI 34.7-51.2), 22.8% (95% CI 21.2-24.4), and 12.8% (95% CI 11.4-14.5), respectively. In multivariable analyses, the highest rate of P. vivax parasitaemia over 42 days of follow-up was in patients residing in areas of short relapse periodicity (adjusted hazard ratio [AHR] = 6.2, 95% CI 2.0-19.5; p = 0.002); patients treated with AL (AHR = 6.2, 95% CI 4.6-8.5; p < 0.001), AA (AHR = 2.3, 95% CI 1.4-3.7; p = 0.001), or AM (AHR = 1.4, 95% CI 1.0-1.9; p = 0.028) compared with DP; and patients who did not clear their initial parasitaemia within 2 days (AHR = 1.8, 95% CI 1.4-2.3; p < 0.001). The analysis was limited by heterogeneity between study populations and lack of data from very low transmission settings. Study quality was high.
Conclusions: In this meta-analysis, we found a high risk of P. vivax parasitaemia after treatment of P. falciparum malaria that varied significantly between studies. These P. vivax infections are likely attributable to relapses that could be prevented with radical cure including a hypnozoitocidal agent; however, the benefits of such a novel strategy will vary considerably between geographical areas.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: RMF is employed currently by AstraZeneca but has no financial stake in the results of the current study. EAA and NJW are Academic Editors on PLOS Medicine's editorial board, and IM was previously an Academic Editor.
Figures



References
-
- World Health Organization. World Malaria Report 2019 Geneva, Switzerland: World Health Organization; 2019. [cited 2020 Aug 26]. Available from: https://www.who.int/publications/i/item/world-malaria-report-2019
-
- World Health Organization. Global technical strategy for malaria 2016–2030 Geneva, Switzerland: World Health Organization; 2015. [cited 2020 Aug 26]. Available from: https://www.who.int/malaria/publications/atoz/9789241564991/en/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

