Large transient capacitive currents in wild-type lysosomal Cl-/H+ antiporter ClC-7 and residual transport activity in the proton glutamate mutant E312A
- PMID: 33211806
- PMCID: PMC7681918
- DOI: 10.1085/jgp.202012583
Large transient capacitive currents in wild-type lysosomal Cl-/H+ antiporter ClC-7 and residual transport activity in the proton glutamate mutant E312A
Abstract
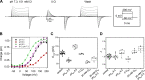
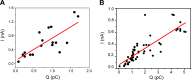
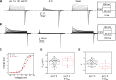
ClC-7 is a lysosomal 2 Cl-/1 H+ antiporter of the CLC protein family, which comprises Cl- channels and other Cl-/H+ antiporters. Mutations in ClC-7 and its associated β subunit Ostm1 lead to osteopetrosis and lysosomal storage disease in humans and mice. Previous studies on other mammalian CLC transporters showed that mutations of a conserved, intracellularly located glutamate residue, the so-called proton glutamate, abolish steady-state transport activity but increase transient capacitive currents associated with partial reactions of the transport cycle. In contrast, we observed large, transient capacitive currents for the wild-type ClC-7, which depend on external pH and internal, but not external, Cl-. Very similar transient currents were observed for the E312A mutant of the proton glutamate. Interestingly, and unlike in other mammalian CLC transporters investigated so far, the E312A mutation strongly reduces, but does not abolish, stationary transport currents, potentially explaining the intermediate phenotype observed in the E312A mouse line.
© 2020 Pusch and Zifarelli.
Figures

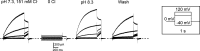




Comment in
-
Not so transport incompetent after all: Revisiting a CLC-7 mutant sheds new mechanistic light on lysosomal physiology.J Gen Physiol. 2021 Apr 5;153(4):e202012805. doi: 10.1085/jgp.202012805. J Gen Physiol. 2021. PMID: 33570555 Free PMC article.

