A new role of glutathione peroxidase 4 during human erythroblast enucleation
- PMID: 33211827
- PMCID: PMC7686909
- DOI: 10.1182/bloodadvances.2020003100
A new role of glutathione peroxidase 4 during human erythroblast enucleation
Abstract
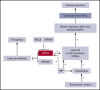
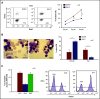
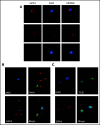
The selenoprotein glutathione peroxidase 4 (GPX4), the only member of the glutathione peroxidase family able to directly reduce cell membrane-oxidized fatty acids and cholesterol, was recently identified as the central regulator of ferroptosis. GPX4 knockdown in mouse hematopoietic cells leads to hemolytic anemia and to increased spleen erythroid progenitor death. The role of GPX4 during human erythropoiesis is unknown. Using in vitro erythroid differentiation, we show here that GPX4-irreversible inhibition by 1S,3R-RSL3 (RSL3) and its short hairpin RNA-mediated knockdown strongly impaired enucleation in a ferroptosis-independent manner not restored by tocopherol or iron chelators. During enucleation, GPX4 localized with lipid rafts at the cleavage furrows between reticulocytes and pyrenocytes. Its inhibition impacted enucleation after nuclear condensation and polarization and was associated with a defect in lipid raft clustering (cholera toxin staining) and myosin-regulatory light-chain phosphorylation. Because selenoprotein translation and cholesterol synthesis share a common precursor, we investigated whether the enucleation defect could represent a compensatory mechanism favoring GPX4 synthesis at the expense of cholesterol, known to be abundant in lipid rafts. Lipidomics and filipin staining failed to show any quantitative difference in cholesterol content after RSL3 exposure. However, addition of cholesterol increased cholera toxin staining and myosin-regulatory light-chain phosphorylation, and improved enucleation despite GPX4 knockdown. In summary, we identified GPX4 as a new actor of human erythroid enucleation, independent of its function in ferroptosis control. We described its involvement in lipid raft organization required for contractile ring assembly and cytokinesis, leading in fine to nucleus extrusion.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





References
-
- Yant LJ, Ran Q, Rao L, et al. . The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34(4):496-502. - PubMed
-
- Forcina GC, Dixon SJ. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics. 2019;19(18):e1800311. - PubMed

