Denosumab Safety and Efficacy Among Participants in the FREEDOM Extension Study With Mild to Moderate Chronic Kidney Disease
- PMID: 33211870
- PMCID: PMC7823314
- DOI: 10.1210/clinem/dgaa851
Denosumab Safety and Efficacy Among Participants in the FREEDOM Extension Study With Mild to Moderate Chronic Kidney Disease
Abstract
Context: The effects of long-term exposure to denosumab in individuals with renal insufficiency are unknown.
Objective: This post hoc analysis evaluates the long-term safety and efficacy of denosumab in individuals with mild-to-moderate chronic kidney disease (CKD) (stages 2 and 3) using data from the pivotal phase 3, double-blind, 3-year FREEDOM (NCT00089791) and open-label, 7-year extension (NCT00523341) studies.
Participants and methods: Women age 60 to 90 years with a bone mineral density (BMD) T-score of less than -2.5 to greater than -4.0 at the total hip or lumbar spine were randomly assigned 1:1 to receive denosumab 60 mg subcutaneously every 6 months (long-term arm) or placebo (cross-over arm) in FREEDOM; eligible participants could enroll in the extension to receive denosumab 60 mg subcutaneously every 6 months. Change in estimated glomerular filtration rate (eGFR) from study baseline and annualized rates of fracture and adverse events (AEs) were the main outcome measures.
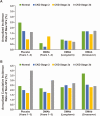
Results: Most participants (1259/1969 [64%] long-term arm; 1173/1781 [66%] crossover arm) with baseline CKD stage 2 or 3 remained within the same CKD subgroup at study completion; less than 3% progressed to CKD stage 4. Participants in all eGFR subgroups showed similar, persistent BMD gains over time and a low incidence of fractures. The percentage of participants reporting serious AEs was similar among renal subgroups (normal, CKD stage 2, CKD stage 3a, CKD stage 3b) both for the long-term (54% vs 52% vs 57% vs 58%) and crossover (43% vs 42% vs 43% vs 68%) arms, except CKD stage 3b subgroup, crossover arm.
Conclusion: The safety and efficacy of denosumab did not differ among participants with mild to moderate CKD.
Keywords: bone mineral density; chronic kidney disease; denosumab; fracture; safety.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


Comment in
-
Letter to the Editor From Zhu and Briganti: "Denosumab Safety and Efficacy Among Participants in the FREEDOM Extension Study With Mild to Moderate Chronic Kidney Disease".J Clin Endocrinol Metab. 2021 Jun 16;106(7):e2833-e2834. doi: 10.1210/clinem/dgab305. J Clin Endocrinol Metab. 2021. PMID: 33954779 No abstract available.
References
-
- Schaeffner ES, Ebert N, Delanaye P, et al. . Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157(7):471-481. - PubMed
-
- Klawansky S, Komaroff E, Cavanaugh PF Jr, et al. . Relationship between age, renal function and bone mineral density in the US population. Osteoporos Int. 2003;14(7):570-576. - PubMed
-
- Dukas LC, Schacht E, Mazor Z, Stähelin HB. A new significant and independent risk factor for falls in elderly men and women: a low creatinine clearance of less than 65 ml/min. Osteoporos Int. 2005;16(3):332-338. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

