Warp-Speed Covid-19 Vaccine Development: Beneficiaries of Maturation in Biopharmaceutical Technologies and Public-Private Partnerships
- PMID: 33212162
- PMCID: PMC7671640
- DOI: 10.1016/j.xphs.2020.11.010
Warp-Speed Covid-19 Vaccine Development: Beneficiaries of Maturation in Biopharmaceutical Technologies and Public-Private Partnerships
Abstract
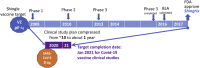
It is anticipated that effective vaccines will enable the resumption of social and economic normalcy. Current calls for masking, social distancing and other restrictive measures for the public-good are difficult to enforce and are unstainable. As ~2-4% of the 50 million SARS-CoV2-infected have succumbed to Covid-19, the US department of Health and Human Services has organized a public-private partnership called Operation Warp Speed (OWS) to develop, produce and deliver 300 million doses of safe and effective vaccines with a January 2021 target. While a majority of the 300+ Covid-19 vaccine candidates are in various stages of preclinical and early-stage clinical testing, 6 clinical candidates are supported with over 10 billion USD plus integrated resources under the OWS agenda. This unprecedented approach is investing in the manufacture of product candidates ahead of product approval. It is enabled by new gene and recombinant pharmaceutical platform technologies that are accelerating the clinical study timeline from ~10 to less than 1 year. It is anticipated that one or more of the 6 candidates under the OWS initiative will be safe, effective and provide a sustained immune response to prevent infection and disease progression. This way, social and economic activities could return to normalcy.
Keywords: Biopharmaceutics; Biotechology; Covid-19; Formulation; Gene therapy; SARS-CoV2; Vaccines.
Copyright © 2020 American Pharmacists Association®. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Halstead S., O'Rourke E. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977:739–741. - PubMed
-
- GSK Shingrix: zoster vaccine recombinant, adjuvanted. 2018. https://www.fda.govhttps://www.fda.gov/media/112428/download - PMC - PubMed
-
- US Health and Human Services-HHS Department of health and human services. 2020. https://www.hhs.gov/sites/default/files/fact-sheet-operation-warp-speed.pdf HHS.gov.
-
- Le T.T., Cramer J.P., Chen R., Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(10):667–668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

