MicroRNAs and fracture healing: Pre-clinical studies
- PMID: 33212318
- PMCID: PMC7769985
- DOI: 10.1016/j.bone.2020.115758
MicroRNAs and fracture healing: Pre-clinical studies
Abstract
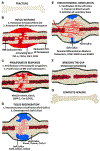
During the past several years, pre-clinical experiments have established that microRNAs (miRNAs), small non-coding RNAs, serve as key regulatory molecules of fracture healing. Their easy modulation with agonists and antagonists make them highly desirable targets for future therapeutic strategies, especially for pathophysiologic fractures that either do not heal (nonunions) or are delayed. It is now well documented that these problematic fractures lead to human suffering and impairment of life quality. Additionally, financial difficulties are also encountered as work productivity decreases and income is reduced. Moreover, targeting miRNAs may also be an avenue to enhancing normal physiological fracture healing. Herein we present the most current knowledge of the involvement of miRNAs during fracture healing in pre-clinical studies. Following a brief description on the nature of miRNAs and of the fracture healing process, we present data from studies focusing specifically, on miRNA regulation of osteoblast differentiation and osteogenesis (within the context of known signaling pathways), chondrocytes, angiogenesis, and apoptosis, all critical to successful bone repair. Further, we also discuss miRNAs and exosomes. We hope that this manuscript serves as a comprehensive review that will facilitate basic/translational scientists in the orthopaedic arena to realize and further decipher the biological and future therapeutic impact of these small regulatory RNA molecules, especially as they relate to the molecular events of each of the major phases of fracture healing.
Keywords: Chondrocytes; Exosomes; Fracture healing; Osteoblasts; miRNAs.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures



References
-
- Initiative: USBaJ The Burden of Musculoskeletal Diseases in the United States (BMUS), Third Edition 2014.
-
- Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z, Steen RG. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg 2016;151: e162775. - PubMed
-
- Force UPST. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA 2018;319: 2521–2531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

