Osteoblastic monocyte chemoattractant protein-1 (MCP-1) mediation of parathyroid hormone's anabolic actions in bone implicates TGF-β signaling
- PMID: 33212319
- PMCID: PMC8628523
- DOI: 10.1016/j.bone.2020.115762
Osteoblastic monocyte chemoattractant protein-1 (MCP-1) mediation of parathyroid hormone's anabolic actions in bone implicates TGF-β signaling
Abstract
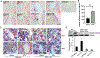
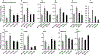
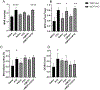
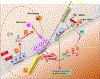
Parathyroid hormone (PTH) is necessary for the regulation of calcium homeostasis and PTH (1-34) was the first approved osteoanabolic therapy for osteoporosis. It is well established that intermittent PTH increases bone formation and that bone remodeling and several cytokines and chemokines play an essential role in this process. Earlier, we had established that the chemokine, monocyte chemoattractant protein-1 (MCP-1/CCL2), was the most highly stimulated gene in rat bone after intermittent PTH injections. Nevertheless, MCP-1 function in bone appears to be complicated. To identify the primary cells expressing MCP-1 in response to PTH, we performed in situ hybridization of rat bone sections after hPTH (1-34) injections and showed that bone-lining osteoblasts are the primary cells that express MCP-1 after PTH treatment. We previously demonstrated MCP-1's importance by showing that PTH's anabolic effects are abolished in MCP-1 null mice, further implicating a role for the chemokine in this process. To establish whether rhMCP-1 peptide treatment could rescue the anabolic effect of PTH in MCP-1 null mice, we treated 4-month-old wild-type (WT) mice with hPTH (1-34) and MCP-1-/- mice with rhMCP-1 and/or hPTH (1-34) for 6 weeks. Micro-computed tomography (μCT) analysis of trabecular and cortical bone showed that MCP-1 injections for 6 weeks rescued the PTH anabolic effect in MCP-1-/- mice. In fact, the combination of rhMCP-1 and hPTH (1-34) has a synergistic anabolic effect compared with monotherapies. Mechanistically, PTH-enhanced transforming growth factor-β (TGF-β) signaling is abolished in the absence of MCP-1, while MCP-1 peptide treatment restores TGF-β signaling in the bone marrow. Here, we have shown that PTH regulates the transcription of the chemokine MCP-1 in osteoblasts and determined how MCP-1 affects bone cell function in PTH's anabolic actions. Taken together, our current work indicates that intermittent PTH stimulates osteoblastic secretion of MCP-1, which leads to increased TGF-β signaling, implicating it in PTH's anabolic actions.
Keywords: Bone; Chemokines; Monocyte chemoattractant protein-1; Osteoporosis; PTH.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
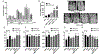
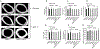
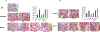
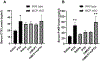
Similar articles
-
Monocyte chemoattractant protein-1 is a mediator of the anabolic action of parathyroid hormone on bone.J Bone Miner Res. 2013 Sep;28(9):1975-86. doi: 10.1002/jbmr.1933. J Bone Miner Res. 2013. PMID: 23519994 Free PMC article.
-
Catabolic Effects of Human PTH (1-34) on Bone: Requirement of Monocyte Chemoattractant Protein-1 in Murine Model of Hyperparathyroidism.Sci Rep. 2017 Nov 10;7(1):15300. doi: 10.1038/s41598-017-15563-7. Sci Rep. 2017. PMID: 29127344 Free PMC article.
-
Mechanisms for the bone anabolic effect of parathyroid hormone treatment in humans.Scand J Clin Lab Invest. 2012 Feb;72(1):14-22. doi: 10.3109/00365513.2011.624631. Epub 2011 Nov 16. Scand J Clin Lab Invest. 2012. PMID: 22085136 Review.
-
Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts.J Biol Chem. 2007 Nov 9;282(45):33098-106. doi: 10.1074/jbc.M611781200. Epub 2007 Aug 9. J Biol Chem. 2007. PMID: 17690108
-
Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) Drives Activation of Bone Remodelling and Skeletal Metastasis.Curr Osteoporos Rep. 2019 Dec;17(6):538-547. doi: 10.1007/s11914-019-00545-7. Curr Osteoporos Rep. 2019. PMID: 31713180 Free PMC article. Review.
Cited by
-
Interplay Between Skeletal and Hematopoietic Cells in the Bone Marrow Microenvironment in Homeostasis and Aging.Curr Osteoporos Rep. 2024 Aug;22(4):416-432. doi: 10.1007/s11914-024-00874-2. Epub 2024 May 23. Curr Osteoporos Rep. 2024. PMID: 38782850 Review.
-
Monocytes and cervical ripening: a narrative review of prolonged labor pathophysiology.Ann Med Surg (Lond). 2025 May 21;87(6):3289-3299. doi: 10.1097/MS9.0000000000003004. eCollection 2025 Jun. Ann Med Surg (Lond). 2025. PMID: 40486626 Free PMC article. Review.
-
Exploring the causal genetic relationship between 41 inflammatory cytokines and osteoporosis: a bidirectional Mendelian randomization analysis.SAGE Open Med. 2025 Jul 29;13:20503121251360176. doi: 10.1177/20503121251360176. eCollection 2025. SAGE Open Med. 2025. PMID: 40756619 Free PMC article.
-
Attenuation of Alzheimer's brain pathology in 5XFAD mice by PTH1-34, a peptide of parathyroid hormone.Alzheimers Res Ther. 2023 Mar 14;15(1):53. doi: 10.1186/s13195-023-01202-z. Alzheimers Res Ther. 2023. PMID: 36918976 Free PMC article.
-
Clofazimine inhibits small-cell lung cancer progression by modulating the kynurenine/aryl hydrocarbon receptor axis.Int J Biol Macromol. 2024 Dec;282(Pt 3):136921. doi: 10.1016/j.ijbiomac.2024.136921. Epub 2024 Oct 28. Int J Biol Macromol. 2024. PMID: 39490481
References
-
- Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R, Anabolic actions of parathyroid hormone on bone, Endocr Rev 14(6) (1993) 690–709. - PubMed
-
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ, Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation, Cell 93(2) (1998) 165–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

