The Role of α-Synuclein Oligomers in Parkinson's Disease
- PMID: 33212758
- PMCID: PMC7697105
- DOI: 10.3390/ijms21228645
The Role of α-Synuclein Oligomers in Parkinson's Disease
Abstract
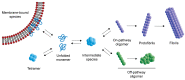
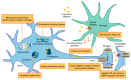
α-synuclein (α-syn) is a protein associated with the pathogenesis of Parkinson's disease (PD), the second most common neurodegeneration disease with no effective treatment. However, how α-syn drives the pathology of PD remains elusive. Recent studies suggest that α-syn oligomers are the primary cause of neurotoxicity and play a critical role in PD. In this review, we discuss the process of α-syn oligomers formation and the current understanding of the structures of oligomers. We also describe seed and propagation effects of oligomeric forms of α-syn. Then, we summarize the mechanism by which α-syn oligomers exert neurotoxicity and promote neurodegeneration, including mitochondrial dysfunction, endoplasmic reticulum stress, proteostasis dysregulation, synaptic impairment, cell apoptosis and neuroinflammation. Finally, we investigate treatment regimens targeting α-syn oligomers at present. Further research is needed to understand the structure and toxicity mechanism of different types of oligomers, so as to provide theoretical basis for the treatment of PD.
Keywords: Parkinson’s disease; oligomers; structure; toxicity; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

