Peripheral Nerve Regeneration and Muscle Reinnervation
- PMID: 33212795
- PMCID: PMC7697710
- DOI: 10.3390/ijms21228652
Peripheral Nerve Regeneration and Muscle Reinnervation
Abstract
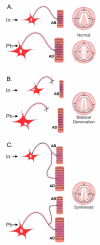
Injured peripheral nerves but not central nerves have the capacity to regenerate and reinnervate their target organs. After the two most severe peripheral nerve injuries of six types, crush and transection injuries, nerve fibers distal to the injury site undergo Wallerian degeneration. The denervated Schwann cells (SCs) proliferate, elongate and line the endoneurial tubes to guide and support regenerating axons. The axons emerge from the stump of the viable nerve attached to the neuronal soma. The SCs downregulate myelin-associated genes and concurrently, upregulate growth-associated genes that include neurotrophic factors as do the injured neurons. However, the gene expression is transient and progressively fails to support axon regeneration within the SC-containing endoneurial tubes. Moreover, despite some preference of regenerating motor and sensory axons to "find" their appropriate pathways, the axons fail to enter their original endoneurial tubes and to reinnervate original target organs, obstacles to functional recovery that confront nerve surgeons. Several surgical manipulations in clinical use, including nerve and tendon transfers, the potential for brief low-frequency electrical stimulation proximal to nerve repair, and local FK506 application to accelerate axon outgrowth, are encouraging as is the continuing research to elucidate the molecular basis of nerve regeneration.
Keywords: Schwann cells; peripheral nerve injuries; peripheral nerve regeneration; regenerating peripheral nerves; skeletal muscle reinnervation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

