Upregulation of iNOS Protects Cyclic Mechanical Stretch-Induced Cell Death in Rat Aorta Smooth Muscle Cells
- PMID: 33212839
- PMCID: PMC7698365
- DOI: 10.3390/ijms21228660
Upregulation of iNOS Protects Cyclic Mechanical Stretch-Induced Cell Death in Rat Aorta Smooth Muscle Cells
Abstract
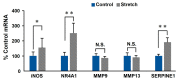
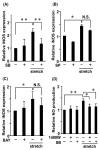
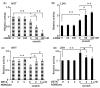
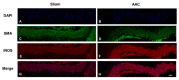
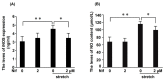
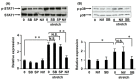
Aortic dissection and aneurysm are associated with abnormal hemodynamic loads originating from hypertension. Our previous study demonstrated that cyclic mechanical stretch (CMS, mimicked hypertension) caused the death of rat aortic smooth muscle cells (RASMCs) in a mitogen activated-protein kinases (MAPKs)-dependent manner. The current study investigated the effects of inducible nitric oxide synthase (iNOS) on CMS-induced RASMC death. cDNA microarrays for CMS-treated RASMCs showed that iNOS expression levels were increased in response to CMS. Real-time polymerase chain reaction (PCR) analysis demonstrated that this increase was p38 MAPK (p38)-dependent. NO production was also increased. This increase could be inhibited by p38 and iNOS inhibitors. Thus, CMS-induced iNOS synthesized NO. CMS-induced cell death in RASMCs was increased by the iNOS inhibitor but abrogated by the long-acting NO donor DETA-NONOate. Increased iNOS expression was confirmed in the abdominal aortic constriction mouse model. Signal transducers and activators of transcription 1 (STAT1) was activated in stretched RASMCs, and iNOS expression and NO production were inhibited by the STAT1 inhibitor nifuroxazide. Our findings suggest that RASMCs were protected by iNOS from CMS-stimulated cell death through the STAT1 and p38 signal pathways independently.
Keywords: NOS; cell death; hypertension; stretch; vascular smooth muscle cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Comprehensive Retrospective Study on the Mechanisms of Cyclic Mechanical Stretch-Induced Vascular Smooth Muscle Cell Death Underlying Aortic Dissection and Potential Therapeutics for Preventing Acute Aortic Aneurysm and Associated Ruptures.Int J Mol Sci. 2024 Feb 22;25(5):2544. doi: 10.3390/ijms25052544. Int J Mol Sci. 2024. PMID: 38473793 Free PMC article. Review.
-
Chemokines protect vascular smooth muscle cells from cell death induced by cyclic mechanical stretch.Sci Rep. 2017 Nov 23;7(1):16128. doi: 10.1038/s41598-017-15867-8. Sci Rep. 2017. PMID: 29170451 Free PMC article.
-
[Vascular smooth muscle cell response to cyclic mechanical stretch and aortic dissection].Nihon Yakurigaku Zasshi. 2018;151(4):155-159. doi: 10.1254/fpj.151.155. Nihon Yakurigaku Zasshi. 2018. PMID: 29628463 Japanese.
-
Azelnidipine inhibits cultured rat aortic smooth muscle cell death induced by cyclic mechanical stretch.PLoS One. 2014 Jul 17;9(7):e102813. doi: 10.1371/journal.pone.0102813. eCollection 2014. PLoS One. 2014. PMID: 25032824 Free PMC article.
-
R59949, a diacylglycerol kinase inhibitor, inhibits inducible nitric oxide production through decreasing transplasmalemmal L-arginine uptake in vascular smooth muscle cells.Naunyn Schmiedebergs Arch Pharmacol. 2017 Feb;390(2):207-214. doi: 10.1007/s00210-016-1316-5. Epub 2016 Dec 1. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 27909743
Cited by
-
The Molecular Mechanism Investigation of HBP-A Slows Down Meniscus Hypertrophy and Mineralisation by the Damage Mechanical Model.J Cell Mol Med. 2024 Dec;28(23):e70271. doi: 10.1111/jcmm.70271. J Cell Mol Med. 2024. PMID: 39656450 Free PMC article.
-
The Phenotypic Responses of Vascular Smooth Muscle Cells Exposed to Mechanical Cues.Cells. 2021 Aug 26;10(9):2209. doi: 10.3390/cells10092209. Cells. 2021. PMID: 34571858 Free PMC article. Review.
-
The Antioxidant/Nitric Oxide-Quenching Agent Cobinamide Prevents Aortic Disease in a Mouse Model of Marfan Syndrome.JACC Basic Transl Sci. 2023 Oct 4;9(1):46-62. doi: 10.1016/j.jacbts.2023.07.014. eCollection 2024 Jan. JACC Basic Transl Sci. 2023. PMID: 38362350 Free PMC article.
-
C/EBPα-Mediated Transcriptional Activation of PIK3C2A Regulates Autophagy, Matrix Metalloproteinase Expression, and Phenotypic of Vascular Smooth Muscle Cells in Aortic Dissection.J Immunol Res. 2022 Sep 12;2022:7465353. doi: 10.1155/2022/7465353. eCollection 2022. J Immunol Res. 2022. PMID: 36132983 Free PMC article.
-
A Comprehensive Retrospective Study on the Mechanisms of Cyclic Mechanical Stretch-Induced Vascular Smooth Muscle Cell Death Underlying Aortic Dissection and Potential Therapeutics for Preventing Acute Aortic Aneurysm and Associated Ruptures.Int J Mol Sci. 2024 Feb 22;25(5):2544. doi: 10.3390/ijms25052544. Int J Mol Sci. 2024. PMID: 38473793 Free PMC article. Review.
References
-
- Guilmet D., Bachet J., Goudot B., Dreyfus G., Martinelli G.L. Aortic dissection: Anatomic types and surgical approaches. J. Cardiovasc. Surg. 1993;34:23–32. - PubMed
-
- Park Y.K., Yi H.J., Lee Y.J., Kim Y.S. Spontaneous Anterior Cerebral Artery Dissection Presenting with Simultaneous Subarachnoid Hemorrhage and Cerebral Infarction in a Patient with Multiple Extracranial Arterial Dissections. J. Korean Neurosurg. Soc. 2013;53:115–117. doi: 10.3340/jkns.2013.53.2.115. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

