I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets
- PMID: 33212847
- PMCID: PMC7698452
- DOI: 10.3390/pharmaceutics12111100
I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets
Abstract
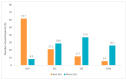
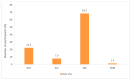
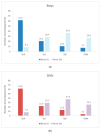
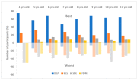
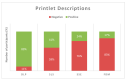
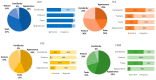
3D printing (3DP) in the pharmaceutical field is a disruptive technology that allows the preparation of personalised medicines at the point of dispensing. The paediatric population presents a variety of pharmaceutical formulation challenges such as dose flexibility, patient compliance, taste masking and the fear or difficulty to swallow tablets, all factors that could be overcome using the adaptable nature of 3DP. User acceptability studies of 3D printed formulations have been previously carried out in adults; however, feedback from children themselves is essential in establishing the quality target product profile towards the development of age-appropriate medicines. The aim of this study was to investigate the preference of children for different 3D printed tablets (Printlets™) as an important precursor to patient acceptability studies. Four different 3DP technologies; digital light processing (DLP), selective laser sintering (SLS), semi-solid extrusion (SSE) and fused deposition modeling (FDM) were used to prepare placebo printlets with similar physical attributes including size and shape. A single-site, two-part survey was completed with participants aged 4-11 years to determine their preference and opinions based on visual inspection of the printlets. A total of 368 participants completed an individual open questionnaire to visually select the best and worst printlet, and 310 participants completed further non-compulsory open questions to elaborate on their choices. Overall, the DLP printlets were the most visually appealing to the children (61.7%) followed by the SLS printlets (21.2%), and with both the FDM (5.4%) and SSE (11.7%) printlets receiving the lowest scores. However, after being informed that the SSE printlets were chewable, the majority of participants changed their selection and favoured this printlet, despite their original choice, in line with children's preference towards chewable dosage forms. Participant age and sex displayed no significant differences in printlet selection. Printlet descriptions were grouped into four distinct categories; appearance, perceived taste, texture and familiarity, and were found to be equally important when creating a quality target product profile for paediatric 3D printed formulations. This study is the first to investigate children's perceptions of printlets, and the findings aim to provide guidance for further development of paediatric-appropriate medicines using different 3DP technologies.
Keywords: 3D printed drug products; MEDIMAKER 3D printing technology; additive manufacturing; pediatrics; personalized pharmaceuticals; printing formulations and dosage forms; printlets; three-dimensional printing; visual preference.
Conflict of interest statement
The authors declare no conflict of interest. The co-authors Simon Gaisford, Abdul W. Basit and Alvaro Goyanes are directors of FabRx Ltd., The Company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Patient acceptability of 3D printed medicines.Int J Pharm. 2017 Sep 15;530(1-2):71-78. doi: 10.1016/j.ijpharm.2017.07.064. Epub 2017 Jul 24. Int J Pharm. 2017. PMID: 28750894 Clinical Trial.
-
Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations.Pharmaceutics. 2021 Jul 21;13(8):1114. doi: 10.3390/pharmaceutics13081114. Pharmaceutics. 2021. PMID: 34452075 Free PMC article.
-
Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron.Pharmaceutics. 2020 Jan 30;12(2):110. doi: 10.3390/pharmaceutics12020110. Pharmaceutics. 2020. PMID: 32019101 Free PMC article.
-
An Overview of 3D Printing Technologies for Soft Materials and Potential Opportunities for Lipid-based Drug Delivery Systems.Pharm Res. 2018 Nov 7;36(1):4. doi: 10.1007/s11095-018-2531-1. Pharm Res. 2018. PMID: 30406349 Review.
-
3D printing: Principles and pharmaceutical applications of selective laser sintering.Int J Pharm. 2020 Aug 30;586:119594. doi: 10.1016/j.ijpharm.2020.119594. Epub 2020 Jul 2. Int J Pharm. 2020. PMID: 32622811 Review.
Cited by
-
High-Precision 3D Printing of Microporous Cochlear Implants for Personalized Local Drug Delivery.J Funct Biomater. 2023 Oct 3;14(10):494. doi: 10.3390/jfb14100494. J Funct Biomater. 2023. PMID: 37888159 Free PMC article.
-
Influence of PEGDA Molecular Weight and Concentration on the In Vitro Release of the Model Protein BSA-FITC from Photo Crosslinked Systems.Pharmaceutics. 2023 Mar 23;15(4):1039. doi: 10.3390/pharmaceutics15041039. Pharmaceutics. 2023. PMID: 37111525 Free PMC article.
-
A framework for conducting clinical trials involving 3D printing of medicines at the point-of-care.Drug Deliv Transl Res. 2025 Sep;15(9):3078-3097. doi: 10.1007/s13346-025-01868-y. Epub 2025 May 9. Drug Deliv Transl Res. 2025. PMID: 40343691 Free PMC article. Review.
-
3D Printed Personalized Colon-targeted Tablets: A Novel Approach in Ulcerative Colitis Management.Curr Drug Deliv. 2024;21(9):1211-1225. doi: 10.2174/1567201821666230915150544. Curr Drug Deliv. 2024. PMID: 37718525 Review.
-
Preliminary Study on the Development of Caffeine Oral Solid Form 3D Printed by Semi-Solid Extrusion for Application in Neonates.AAPS PharmSciTech. 2023 May 24;24(5):122. doi: 10.1208/s12249-023-02582-z. AAPS PharmSciTech. 2023. PMID: 37225888
References
-
- Basit A.W., Gaisford S. 3d Printing of Pharmaceuticals. Volume 31. Springer; Berlin/Heidelberg, Germany: 2018. - DOI
-
- Aprecia Pharmaceuticals Fda Approves the First 3d Printed Drug Product. [(accessed on 8 September 2020)]; Available online: https://www.aprecia.com/news/fda-approves-the-first-3d-printed-drug-product.
-
- Goyanes A., Madla C.M., Umerji A., Piñeiro G.D., Montero J.M.G., Diaz M.J.L., Barcia M.G., Taherali F., Sánchez-Pintos P., Couce M.-L. Automated therapy preparation of isoleucine formulations using 3d printing for the treatment of msud: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019;567:118497. doi: 10.1016/j.ijpharm.2019.118497. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

