A Perspective on Cell Therapy and Cancer Vaccine in Biliary Tract Cancers (BTCs)
- PMID: 33212880
- PMCID: PMC7698436
- DOI: 10.3390/cancers12113404
A Perspective on Cell Therapy and Cancer Vaccine in Biliary Tract Cancers (BTCs)
Abstract
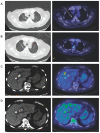
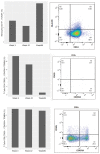
Biliary tract cancer (BTC) is a rare, but aggressive, disease that comprises of gallbladder carcinoma, intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma, with heterogeneous molecular profiles. Advanced disease has limited therapeutic options beyond first-line platinum-based chemotherapy. Immunotherapy has emerged as a viable option for many cancers with a similar unmet need. Therefore, we reviewed current understanding of the tumor immune microenvironment and recent advances in cellular immunotherapy and therapeutic cancer vaccines against BTC. We illustrated the efficacy of dendritic cell vaccination in one patient with advanced, chemorefractory, melanoma-associated antigen (MAGE)-positive gallbladder carcinoma, who was given multiple injections of an allogenic MAGE antigen-positive melanoma cell lysate (MCL)-based autologous dendritic cell vaccine combined with sequential anti-angiogenic therapy. This resulted in good radiological and tumor marker response and an overall survival of 3 years from diagnosis. We postulate the potential synergism of adding anti-angiogenic therapy, such as bevacizumab, to immunotherapy in BTC, as a rational scientific principle to positively modulate the tumor microenvironment to augment antitumor immunity.
Keywords: bevacizumab; biliary tract cancer; cell therapy; dendritic cell vaccine; immunotherapy; personalized medicine; review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies.Cancers (Basel). 2023 Jun 23;15(13):3312. doi: 10.3390/cancers15133312. Cancers (Basel). 2023. PMID: 37444422 Free PMC article. Review.
-
Immunotherapy for the treatment of biliary tract cancer: an evolving landscape.Ther Adv Med Oncol. 2024 Mar 5;16:17588359241235799. doi: 10.1177/17588359241235799. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 38449562 Free PMC article. Review.
-
Current and emerging immunotherapeutic approaches for biliary tract cancers.Hepatobiliary Pancreat Dis Int. 2022 Oct;21(5):440-449. doi: 10.1016/j.hbpd.2022.08.015. Epub 2022 Sep 7. Hepatobiliary Pancreat Dis Int. 2022. PMID: 36115807 Review.
-
IMbrave 151: a randomized phase II trial of atezolizumab combined with bevacizumab and chemotherapy in patients with advanced biliary tract cancer.Ther Adv Med Oncol. 2021 Jul 31;13:17588359211036544. doi: 10.1177/17588359211036544. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 34377158 Free PMC article.
-
Immunotherapy as a treatment for biliary tract cancers: A review of approaches with an eye to the future.Curr Probl Cancer. 2018 Jan-Feb;42(1):49-58. doi: 10.1016/j.currproblcancer.2017.10.004. Epub 2017 Oct 31. Curr Probl Cancer. 2018. PMID: 29501212 Free PMC article. Review.
Cited by
-
Evolving Role of Immunotherapy in Advanced Biliary Tract Cancers.Cancers (Basel). 2022 Mar 29;14(7):1748. doi: 10.3390/cancers14071748. Cancers (Basel). 2022. PMID: 35406520 Free PMC article. Review.
-
Immunotherapy as a Therapeutic Strategy for Gastrointestinal Cancer-Current Treatment Options and Future Perspectives.Int J Mol Sci. 2022 Jun 15;23(12):6664. doi: 10.3390/ijms23126664. Int J Mol Sci. 2022. PMID: 35743107 Free PMC article. Review.
-
Chimeric immune checkpoint protein vaccines inhibit the tumorigenesis and growth of rat cholangiocarcinoma.Front Immunol. 2022 Oct 20;13:982196. doi: 10.3389/fimmu.2022.982196. eCollection 2022. Front Immunol. 2022. PMID: 36341387 Free PMC article.
-
An Insight into the Novel Immunotherapy and Targeted Therapeutic Strategies for Hepatocellular Carcinoma and Cholangiocarcinoma.Life (Basel). 2022 Apr 30;12(5):665. doi: 10.3390/life12050665. Life (Basel). 2022. PMID: 35629333 Free PMC article. Review.
-
Discovery of lung adenocarcinoma tumor antigens and ferroptosis subtypes for developing mRNA vaccines.Sci Rep. 2024 Feb 8;14(1):3219. doi: 10.1038/s41598-024-53622-y. Sci Rep. 2024. PMID: 38331967 Free PMC article.
References
-
- Morizane C., Okusaka T., Mizusawa J., Katayama H., Ueno M., Ikeda M., Ozaka M., Okano N., Sugimori K., Fukutomi A., et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 2019;30:1950–1958. doi: 10.1093/annonc/mdz402. - DOI - PubMed
-
- Sakai D., Kanai M., Kobayashi S., Eguchi H., Baba H., Seo S., Taketomi A., Takayama T., Yamaue H., Ishioka C., et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA) Ann. Oncol. 2018;9:1069–1072. doi: 10.1093/annonc/mdy282. - DOI - PMC - PubMed
-
- Shroff R.T., Javle M.M., Xiao L., Kaseb A.O., Varadhachary G.R., Wolff R.A., Raghav K.P.S., Iwasaki M., Masci P., Ramanathan R.K., et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824–830. doi: 10.1001/jamaoncol.2019.0270. - DOI - PMC - PubMed
-
- Lamarca A., Palmer D.H., Wasan H.S., Ross P.J., Ma Y.T., Arora A., Falk S., Gillmore R., Wadsley J., Patel K., et al. A randomised phase III, multi-centre, open-label study of Active Symptom Control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J. Clin. Oncol. 2019;37(Suppl. 15):4003.
Publication types
LinkOut - more resources
Full Text Sources

