Microneedles Drug Delivery Systems for Treatment of Cancer: A Recent Update
- PMID: 33212921
- PMCID: PMC7698361
- DOI: 10.3390/pharmaceutics12111101
Microneedles Drug Delivery Systems for Treatment of Cancer: A Recent Update
Abstract
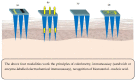
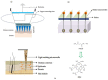
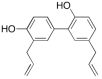
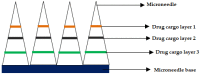
Microneedles (MNs) are tiny needle like structures used in drug delivery through layers of the skin. They are non-invasive and are associated with significantly less or no pain at the site of administration to the skin. MNs are excellent in delivering both small and large molecules to the subjects in need thereof. There exist several strategies for drug delivery using MNs, wherein each strategy has its pros and cons. Research in this domain lead to product development and commercialization for clinical use. Additionally, several MN-based products are undergoing clinical trials to evaluate its safety, efficacy, and tolerability. The present review begins by providing bird's-eye view about the general characteristics of MNs followed by providing recent updates in the treatment of cancer using MNs. Particularly, we provide an overview of various aspects namely: anti-cancerous MNs that work based on sensor technology, MNs for treatment of breast cancer, skin carcinoma, prostate cancer, and MNs fabricated by additive manufacturing or 3 dimensional printing for treatment of cancer. Further, the review also provides limitations, safety concerns, and latest updates about the clinical trials on MNs for the treatment of cancer. Furthermore, we also provide a regulatory overview from the "United States Food and Drug Administration" about MNs.
Keywords: breast cancer; non-invasive; regulatory; skin carcinoma; transdermal drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
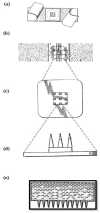
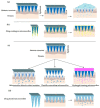
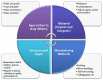
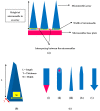
References
-
- Andersen O., Zweidorff O.K., Hjelde T., Rodland E.A. Problemer med å svelgetabletter. Spørreundersøkelse fra allmennpraksis. Problems when swallowing tablets. A questionnaire study from general practice. TidsskrNorLaegeforen. 1995;115:947–949. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

