Highly Red Light-Emitting Erbium- and Lutetium-Doped Core-Shell Upconverting Nanoparticles Surface-Modified with PEG-Folic Acid/TCPP for Suppressing Cervical Cancer HeLa Cells
- PMID: 33212942
- PMCID: PMC7698343
- DOI: 10.3390/pharmaceutics12111102
Highly Red Light-Emitting Erbium- and Lutetium-Doped Core-Shell Upconverting Nanoparticles Surface-Modified with PEG-Folic Acid/TCPP for Suppressing Cervical Cancer HeLa Cells
Abstract
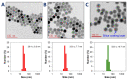
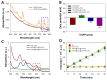
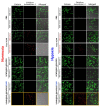
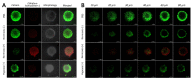
Photodynamic therapy (PDT) combined with upconverting nanoparticles (UCNPs) are viewed together as an effective method of ablating tumors. After absorbing highly tissue-penetrating near-infrared (NIR) light, UCNPs emit a shorter wavelength light (~660 nm) suitable for PDT. In this study, we designed and prepared highly red fluorescence-emitting silica-coated core-shell upconverting nanoparticles modified with polyethylene glycol (PEG5k)-folic acid and tetrakis(4-carboxyphenyl)porphyrin (TCPP) (UCNPs@SiO2-NH2@FA/PEG/TCPP) as an efficient photodynamic agent for killing tumor cells. The UCNPs consisted of two simple lanthanides, erbium and lutetium, as the core and shell, respectively. The unique core-shell combination enabled the UCNPs to emit red light without green light. TCPP, folic acid, and PEG were conjugated to the outer silica layer of UCNPs as a photosensitizing agent, a ligand for tumor attachment, and a dispersing stabilizer, respectively. The prepared UCNPs of ~50 nm diameter and -34.5 mV surface potential absorbed 808 nm light and emitted ~660 nm red light. Most notably, these UCNPs were physically well dispersed and stable in the aqueous phase due to PEG attachment and were able to generate singlet oxygen (1O2) with a high efficacy. The HeLa cells were treated with each UCNP sample (0, 1, 5, 10, 20, 30 μg/mL as a free TCPP). The results showed that the combination of UCNPs@SiO2-NH2@FA/PEG/TCPP and the 808 nm laser was significantly cytotoxic to HeLa cells, almost to the same degree as naïve TCPP plus the 660 nm laser based on MTT and Live/Dead assays. Furthermore, the UCNPs@SiO2-NH2@FA/PEG/TCPP was well internalized into HeLa cells and three-dimensional HeLa spheroids, presumably due to the surface folic acid and small size in conjunction with endocytosis and the nonspecific uptake. We believe that our UCNPs@SiO2-NH2@FA/PEG/TCPP will serve as a new platform for highly efficient and deep-penetrating photodynamic agents suitable for various tumor treatments.
Keywords: cancer; hypoxia; near infrared; photodynamic therapy; tetrakis(4-carboxy-phenyl)porphyrin; upconverting nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Photosensitiser functionalised luminescent upconverting nanoparticles for efficient photodynamic therapy of breast cancer cells.Photochem Photobiol Sci. 2019 Jan 1;18(1):98-109. doi: 10.1039/c8pp00354h. Epub 2018 Oct 17. Photochem Photobiol Sci. 2019. PMID: 30328457
-
Macrophage-reprogramming upconverting nanoparticles for enhanced TAM-mediated antitumor therapy of hypoxic breast cancer.J Control Release. 2023 Aug;360:482-495. doi: 10.1016/j.jconrel.2023.07.009. Epub 2023 Jul 12. J Control Release. 2023. PMID: 37423526
-
Monodisperse Core-Shell NaYF4:Yb3+/Er3+@NaYF4:Nd3+-PEG-GGGRGDSGGGY-NH2 Nanoparticles Excitable at 808 and 980 nm: Design, Surface Engineering, and Application in Life Sciences.Front Chem. 2020 Jun 12;8:497. doi: 10.3389/fchem.2020.00497. eCollection 2020. Front Chem. 2020. PMID: 32596210 Free PMC article.
-
A comprehensive review on singlet oxygen generation in nanomaterials and conjugated polymers for photodynamic therapy in the treatment of cancer.Nanoscale. 2024 Feb 15;16(7):3243-3268. doi: 10.1039/d3nr05801h. Nanoscale. 2024. PMID: 38265094 Review.
-
Aminosilane Functionalization and Cytotoxicity Effects of Upconversion Nanoparticles Y2O3 and Gd2O3 Co-Doped with Yb3+and Er3.Nanobiomedicine (Rij). 2016 Jan 1;3:1. doi: 10.5772/62252. eCollection 2016 Jan-Dec. Nanobiomedicine (Rij). 2016. PMID: 29942376 Free PMC article. Review.
Cited by
-
Targeted Endoradiotherapy with Lu2O3-iPSMA/-iFAP Nanoparticles Activated by Neutron Irradiation: Preclinical Evaluation and First Patient Image.Pharmaceutics. 2022 Mar 27;14(4):720. doi: 10.3390/pharmaceutics14040720. Pharmaceutics. 2022. PMID: 35456554 Free PMC article.
-
Synthesis of Upconversion Nanoparticles with Manipulable Crystal Phase, Size Specification, and Morphological Features.J Fluoresc. 2025 Aug 13. doi: 10.1007/s10895-025-04476-8. Online ahead of print. J Fluoresc. 2025. PMID: 40802123
-
Upconverting nanoparticle-containing erythrocyte-sized hemoglobin microgels that generate heat, oxygen and reactive oxygen species for suppressing hypoxic tumors.Bioact Mater. 2022 Sep 28;22:112-126. doi: 10.1016/j.bioactmat.2022.09.020. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36203958 Free PMC article.
-
Concerning influences of micro/nano plastics on female reproductive health: focusing on cellular and molecular pathways from animal models to human studies.Reprod Biol Endocrinol. 2024 Nov 11;22(1):141. doi: 10.1186/s12958-024-01314-7. Reprod Biol Endocrinol. 2024. PMID: 39529078 Free PMC article. Review.
-
Engineered upconversion nanoparticles for breast cancer theranostics.Theranostics. 2025 Jul 25;15(16):8259-8319. doi: 10.7150/thno.116153. eCollection 2025. Theranostics. 2025. PMID: 40860139 Free PMC article. Review.
References
-
- Phuong P.T.T., Lee S., Lee C., Seo B., Park S., Oh K.T., Lee E.S., Choi H.-G., Shin B.S., Youn Y.S. Beta-carotene-bound albumin nanoparticles modified with chlorin e6 for breast tumor ablation based on photodynamic therapy. Colloids Surf. B Biointerfaces. 2018;171:123–133. doi: 10.1016/j.colsurfb.2018.07.016. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

