Transthyretin Stabilization: An Emerging Strategy for the Treatment of Alzheimer's Disease?
- PMID: 33212973
- PMCID: PMC7698513
- DOI: 10.3390/ijms21228672
Transthyretin Stabilization: An Emerging Strategy for the Treatment of Alzheimer's Disease?
Abstract
Transthyretin (TTR), previously named prealbumin is a plasma protein secreted mainly by the liver and choroid plexus (CP) that is a carrier for thyroid hormones (THs) and retinol (vitamin A). The structure of TTR, with four monomers rich in β-chains in a globular tetrameric protein, accounts for the predisposition of the protein to aggregate in fibrils, leading to a rare and severe disease, namely transthyretin amyloidosis (ATTR). Much effort has been made and still is required to find new therapeutic compounds that can stabilize TTR ("kinetic stabilization") and prevent the amyloid genetic process. Moreover, TTR is an interesting therapeutic target for neurodegenerative diseases due to its recognized neuroprotective properties in the cognitive impairment context and interestingly in Alzheimer's disease (AD). Much evidence has been collected regarding the neuroprotective effects in AD, including through in vitro and in vivo studies as well as a wide range of clinical series. Despite this supported hypothesis of neuroprotection for TTR, the mechanisms are still not completely clear. The aim of this review is to highlight the most relevant findings on the neuroprotective role of TTR, and to summarize the recent progress on the development of TTR tetramer stabilizers.
Keywords: Alzheimer’s disease; TTR stabilizers; amyloidosis; protein aggregation; protein misfolding; transthyretin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
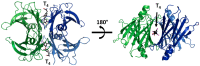
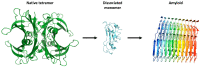
Similar articles
-
[Transthyretin-related amyloidotic cardiomyopathy: looking for the etiological treatment].G Ital Cardiol (Rome). 2014 May;15(5):293-300. doi: 10.1714/1563.17027. G Ital Cardiol (Rome). 2014. PMID: 25002169 Review. Italian.
-
Energetic characteristics of the new transthyretin variant A25T may explain its atypical central nervous system pathology.Lab Invest. 2003 Mar;83(3):409-17. doi: 10.1097/01.lab.0000059937.11023.1f. Lab Invest. 2003. PMID: 12649341
-
The importance of a gatekeeper residue on the aggregation of transthyretin: implications for transthyretin-related amyloidoses.J Biol Chem. 2014 Oct 10;289(41):28324-37. doi: 10.1074/jbc.M114.563981. Epub 2014 Aug 1. J Biol Chem. 2014. PMID: 25086037 Free PMC article.
-
Tolcapone, a potent aggregation inhibitor for the treatment of familial leptomeningeal amyloidosis.FEBS J. 2021 Jan;288(1):310-324. doi: 10.1111/febs.15339. Epub 2020 May 11. FEBS J. 2021. PMID: 32324953
-
Undiscovered Roles for Transthyretin: From a Transporter Protein to a New Therapeutic Target for Alzheimer's Disease.Int J Mol Sci. 2020 Mar 18;21(6):2075. doi: 10.3390/ijms21062075. Int J Mol Sci. 2020. PMID: 32197355 Free PMC article. Review.
Cited by
-
Erythrocytes Are an Independent Protective Factor for Vascular Cognitive Impairment in Patients With Severe White Matter Hyperintensities.Front Aging Neurosci. 2022 Feb 18;14:789602. doi: 10.3389/fnagi.2022.789602. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35250538 Free PMC article.
-
New Hybrid Compounds Incorporating Natural Products as Multifunctional Agents against Alzheimer's Disease.Pharmaceutics. 2023 Sep 22;15(10):2369. doi: 10.3390/pharmaceutics15102369. Pharmaceutics. 2023. PMID: 37896129 Free PMC article.
-
Does the Type of Semen Affect the Phosphoproteome of Turkey (Meleagris gallopavo) Spermatozoa?Int J Mol Sci. 2025 Apr 8;26(8):3467. doi: 10.3390/ijms26083467. Int J Mol Sci. 2025. PMID: 40331949 Free PMC article.
-
Primary Hypothyroidism and Alzheimer's Disease: A Tale of Two.Cell Mol Neurobiol. 2023 Oct;43(7):3405-3416. doi: 10.1007/s10571-023-01392-y. Epub 2023 Aug 4. Cell Mol Neurobiol. 2023. PMID: 37540395 Free PMC article. Review.
-
Approaches for Increasing Cerebral Efflux of Amyloid-β in Experimental Systems.J Alzheimers Dis. 2024;100(2):379-411. doi: 10.3233/JAD-240212. J Alzheimers Dis. 2024. PMID: 38875041 Free PMC article. Review.
References
-
- Soprano D.R., Herbert J., Soprano K.J., Schon E.A., Goodman D.S. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J. Biol. Chem. 1985;260:11793–11798. - PubMed
-
- Raz A., Goodman D.S. The interaction of thyroxine with human plasma prealbumin and with the prealbumin-retinol-binding protein complex. J. Biol. Chem. 1969;244:3230–3237. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

