Transthyretin Stabilization: An Emerging Strategy for the Treatment of Alzheimer's Disease?
- PMID: 33212973
- PMCID: PMC7698513
- DOI: 10.3390/ijms21228672
Transthyretin Stabilization: An Emerging Strategy for the Treatment of Alzheimer's Disease?
Abstract
Transthyretin (TTR), previously named prealbumin is a plasma protein secreted mainly by the liver and choroid plexus (CP) that is a carrier for thyroid hormones (THs) and retinol (vitamin A). The structure of TTR, with four monomers rich in β-chains in a globular tetrameric protein, accounts for the predisposition of the protein to aggregate in fibrils, leading to a rare and severe disease, namely transthyretin amyloidosis (ATTR). Much effort has been made and still is required to find new therapeutic compounds that can stabilize TTR ("kinetic stabilization") and prevent the amyloid genetic process. Moreover, TTR is an interesting therapeutic target for neurodegenerative diseases due to its recognized neuroprotective properties in the cognitive impairment context and interestingly in Alzheimer's disease (AD). Much evidence has been collected regarding the neuroprotective effects in AD, including through in vitro and in vivo studies as well as a wide range of clinical series. Despite this supported hypothesis of neuroprotection for TTR, the mechanisms are still not completely clear. The aim of this review is to highlight the most relevant findings on the neuroprotective role of TTR, and to summarize the recent progress on the development of TTR tetramer stabilizers.
Keywords: Alzheimer’s disease; TTR stabilizers; amyloidosis; protein aggregation; protein misfolding; transthyretin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
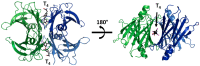
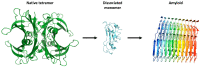
References
-
- Soprano D.R., Herbert J., Soprano K.J., Schon E.A., Goodman D.S. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J. Biol. Chem. 1985;260:11793–11798. - PubMed
-
- Raz A., Goodman D.S. The interaction of thyroxine with human plasma prealbumin and with the prealbumin-retinol-binding protein complex. J. Biol. Chem. 1969;244:3230–3237. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

