Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics
- PMID: 33212974
- PMCID: PMC7698489
- DOI: 10.3390/nano10112276
Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics
Abstract
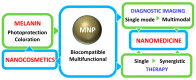
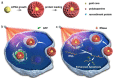
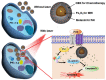
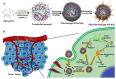
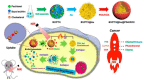
Bioinspired nanomaterials are ideal components for nanomedicine, by virtue of their expected biocompatibility or even complete lack of toxicity. Natural and artificial melanin-based nanoparticles (MNP), including polydopamine nanoparticles (PDA NP), excel for their extraordinary combination of additional optical, electronic, chemical, photophysical, and photochemical properties. Thanks to these features, melanin plays an important multifunctional role in the design of new platforms for nanomedicine where this material works not only as a mechanical support or scaffold, but as an active component for imaging, even multimodal, and simple or synergistic therapy. The number of examples of bio-applications of MNP increased dramatically in the last decade. Here, we review the most recent ones, focusing on the multiplicity of functions that melanin performs in theranostics platforms with increasing complexity. For the sake of clarity, we start analyzing briefly the main properties of melanin and its derivative as well as main natural sources and synthetic methods, moving to imaging application from mono-modal (fluorescence, photoacoustic, and magnetic resonance) to multi-modal, and then to mono-therapy (drug delivery, anti-oxidant, photothermal, and photodynamic), and finally to theranostics and synergistic therapies, including gene- and immuno- in combination to photothermal and photodynamic. Nanomedicine aims not only at the treatment of diseases, but also to their prevention, and melanin in nature performs a protective action, in the form of nanopigment, against UV-Vis radiations and oxidants. With these functions being at the border between nanomedicine and cosmetics nanotechnology, recently examples of applications of artificial MNP in cosmetics are increasing, paving the road to the birth of the new science of nanocosmetics. In the last part of this review, we summarize and discuss these important recent results that establish evidence of the interconnection between nanomedicine and cosmetics nanotechnology.
Keywords: biocompatible; bioimaging; drug delivery; melanin; nanomedicine; nanotoxicity; photodynamic therapy; photothermal therapy; polydopamine; theranostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Melanin for Photoprotection and Hair Coloration in the Emerging Era of Nanocosmetics.Int J Mol Sci. 2024 May 28;25(11):5862. doi: 10.3390/ijms25115862. Int J Mol Sci. 2024. PMID: 38892049 Free PMC article. Review.
-
Melanin-like nanoparticles: advances in surface modification and tumour photothermal therapy.J Nanobiotechnology. 2022 Nov 19;20(1):485. doi: 10.1186/s12951-022-01698-x. J Nanobiotechnology. 2022. PMID: 36402976 Free PMC article. Review.
-
Recent advances in melanin-like nanomaterials in biomedical applications: a mini review.Biomater Res. 2019 Dec 3;23:24. doi: 10.1186/s40824-019-0175-9. eCollection 2019. Biomater Res. 2019. PMID: 31827881 Free PMC article. Review.
-
Natural melanin: from biological functions to biofunctionalized nanoparticles in advanced biomedicine.Biomater Adv. 2025 Nov;176:214368. doi: 10.1016/j.bioadv.2025.214368. Epub 2025 Jun 3. Biomater Adv. 2025. PMID: 40472781 Review.
-
Melanin-Like Nanomedicine in Photothermal Therapy Applications.Int J Mol Sci. 2021 Jan 1;22(1):399. doi: 10.3390/ijms22010399. Int J Mol Sci. 2021. PMID: 33401518 Free PMC article. Review.
Cited by
-
Preclinical pharmaco-toxicological screening of biomimetic melanin-like nanoparticles as a potential therapeutic strategy for cutaneous melanoma.Front Pharmacol. 2025 Feb 6;16:1487854. doi: 10.3389/fphar.2025.1487854. eCollection 2025. Front Pharmacol. 2025. PMID: 39981176 Free PMC article.
-
An efficient ultrasonic-assisted bleaching strategy customized for yak hair triggered by melanin-targeted Fenton reaction.Ultrason Sonochem. 2022 May;86:106020. doi: 10.1016/j.ultsonch.2022.106020. Epub 2022 Apr 29. Ultrason Sonochem. 2022. PMID: 35504136 Free PMC article.
-
Controlled Deposition of Nanostructured Hierarchical TiO2 Thin Films by Low Pressure Supersonic Plasma Jets.Nanomaterials (Basel). 2022 Feb 3;12(3):533. doi: 10.3390/nano12030533. Nanomaterials (Basel). 2022. PMID: 35159878 Free PMC article.
-
The interactions between melatonin and the renin-angiotensin system (RAS) in vascular attenuation in diabetic and non-diabetic conditions.Acta Diabetol. 2025 Jun;62(6):801-809. doi: 10.1007/s00592-025-02479-2. Epub 2025 Mar 13. Acta Diabetol. 2025. PMID: 40080199 Review.
-
Melanin and Melanin-Functionalized Nanoparticles as Promising Tools in Cancer Research-A Review.Cancers (Basel). 2022 Apr 6;14(7):1838. doi: 10.3390/cancers14071838. Cancers (Basel). 2022. PMID: 35406610 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

