Extracellular ATP: A Feasible Target for Cancer Therapy
- PMID: 33212982
- PMCID: PMC7698494
- DOI: 10.3390/cells9112496
Extracellular ATP: A Feasible Target for Cancer Therapy
Abstract
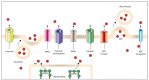
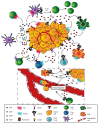
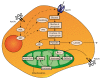
Adenosine triphosphate (ATP) is one of the main biochemical components of the tumor microenvironment (TME), where it can promote tumor progression or tumor suppression depending on its concentration and on the specific ecto-nucleotidases and receptors expressed by immune and cancer cells. ATP can be released from cells via both specific and nonspecific pathways. A non-regulated release occurs from dying and damaged cells, whereas active release involves exocytotic granules, plasma membrane-derived microvesicles, specific ATP-binding cassette (ABC) transporters and membrane channels (connexin hemichannels, pannexin 1 (PANX1), calcium homeostasis modulator 1 (CALHM1), volume-regulated anion channels (VRACs) and maxi-anion channels (MACs)). Extracellular ATP acts at P2 purinergic receptors, among which P2X7R is a key mediator of the final ATP-dependent biological effects. Over the years, P2 receptor- or ecto-nucleotidase-targeting for cancer therapy has been proposed and actively investigated, while comparatively fewer studies have explored the suitability of TME ATP as a target. In this review, we briefly summarize the available evidence suggesting that TME ATP has a central role in determining tumor fate and is, therefore, a suitable target for cancer therapy.
Keywords: cancer; extracellular ATP; purinergic signaling; tumor microenvironment.
Conflict of interest statement
Francesco Di Virgilio is a member of the Scientific Advisory Board of Biosceptre Ltd., a UK-based company involved in the development of P2X7-targeted antibodies. The other authors declare no conflicts of interest.
Figures

References
-
- Burnstock G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

