A Retrospective Analysis about Frequency of Monitoring in Italian Chronic Myeloid Leukemia Patients after Discontinuation
- PMID: 33213044
- PMCID: PMC7698481
- DOI: 10.3390/jcm9113692
A Retrospective Analysis about Frequency of Monitoring in Italian Chronic Myeloid Leukemia Patients after Discontinuation
Abstract
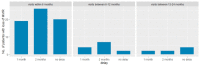
Successful discontinuation of tyrosine kinase inhibitors has been achieved in patients with chronic-phase chronic myeloid leukemia (CML). Careful molecular monitoring after discontinuation warrants safe and prompt resumption of therapy. We retrospectively evaluated how molecular monitoring has been conducted in Italy in a cohort of patients who discontinued tyrosine kinase inhibitor (TKI) treatment per clinical practice. The outcome of these patients has recently been reported-281 chronic-phase CML patients were included in this subanalysis. Median follow-up since discontinuation was 2 years. Overall, 2203 analyses were performed, 17.9% in the first three months and 38.4% in the first six months. Eighty-six patients lost major molecular response (MMR) in a mean time of 5.7 months-65 pts (75.6%) during the first six months. We evaluated the number of patients who would experience a delay in diagnosis of MMR loss if a three-month monitoring schedule was adopted. In the first 6 months, 19 pts (29.2%) would have a one-month delay, 26 (40%) a 2-month delay. Very few patients would experience a delay in the following months. A less intense frequency of monitoring, particularly after the first 6 months off treatment, would not have affected the success of treatment-free remission (TFR) nor put patients at risk of progression.
Keywords: chronic myeloid leukemia; molecular monitoring; treatment-free remission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Etienne G., Guilhot J., Rea D., Rigal-Huguet F., Nicolini F., Charbonnier A., Guerci-Bresler A., Legros L., Varet B., Gardembas M., et al. Long-Term Follow-up of the French Stop Imatinib (STIM1) Study in Patients with Chronic Myeloid Leukemia. J. Clin. Oncol. 2017;35:298–305. doi: 10.1200/JCO.2016.68.2914. - DOI - PubMed
-
- Campiotti L., Suter M.B., Guasti L., Piazza R., Gambacorti-Passerini C., Grandi A.M., Squizzato A. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR-ABL transcript level: A systematic review and a meta-analysis. Eur. J. Cancer. 2017;77:48–56. doi: 10.1016/j.ejca.2017.02.028. - DOI - PubMed
-
- Fava C., Rege-Cambrin G., Dogliotti I., Cerrano M., Berchialla P., Dragani M., Rosti G., Castagnetti F., Gugliotta G., Martino B., et al. Observational study of chronic myeloid leukemia Italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica. 2019;104:1589–1596. doi: 10.3324/haematol.2018.205054. - DOI - PMC - PubMed
-
- Saussele S., Richter J., Guilhot J., Gruber F.X., Hjorth-Hansen H., Almeida A., Mjanssen J.J.W., Mayer J., Koskenvesa P., Panayiotidis P., et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–757. doi: 10.1016/S1470-2045(18)30192-X. - DOI - PubMed
-
- Rea D., Nicolini F.E., Tulliez M., Guilhot F., Guilhot J., Guerci-Bresler A., Gardembas M., Coiteux V., Guillerm G., Legros L., et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: Interim analysis of the STOP 2G-TKI study. Blood. 2017;129:846–854. doi: 10.1182/blood-2016-09-742205. - DOI - PubMed