Lysosomal targeting of autophagosomes by the TECPR domain of TECPR2
- PMID: 33213269
- PMCID: PMC8525938
- DOI: 10.1080/15548627.2020.1852727
Lysosomal targeting of autophagosomes by the TECPR domain of TECPR2
Abstract
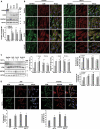
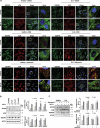
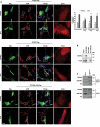
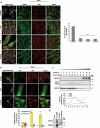
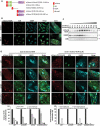
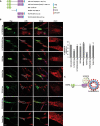
TECPR2 (tectonin beta-propeller repeat containing 2) is a large, multi-domain protein comprised of an amino-terminal WD domain, a middle unstructured region and a carboxy-terminal TEPCR domain comprises of six TECPR repeats followed by a functional LIR motif. Human TECPR2 mutations are linked to spastic paraplegia type 49 (SPG49), a hereditary neurodegenerative disorder. Here we show that basal macroautophagic/autophagic flux is impaired in SPG49 patient fibroblasts in the form of accumulated autophagosomes. Ectopic expression of either full length TECPR2 or the TECPR domain rescued autophagy in patient fibroblasts in a LIR-dependent manner. Moreover, this domain is recruited to the cytosolic leaflet of autophagosomal and lysosomal membranes in a LIR- and VAMP8-dependent manner, respectively. These findings provide evidence for a new role of the TECPR domain in particular, and TECPR2 in general, in lysosomal targeting of autophagosomes via association with Atg8-family proteins on autophagosomes and VAMP8 on lysosomes.Abbreviations: HOPS: homotypic fusion and vacuole protein sorting; LIR: LC3-interacting region; SPG49: spastic paraplegia type 49; STX17: syntaxin 17; TECPR2: tectonin beta-propeller repeat containing 2; VAMP8: vesicle associated membrane protein 8.
Keywords: Autophagy; SPG49; TECPR2; lysosome; neurodegeneration.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
-
- Weidberg H, Shvets E, Elazar Z.. Biogenesis and cargo selectivity of autophagosomes. Annu Rev Biochem. 2011;80:125–156. - PubMed
-
- Ravikumar B, Sarkar S, Davies JE, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010. Oct;90(4):1383–1435. - PubMed
-
- Fraiberg M, Elazar Z. Genetic defects of autophagy linked to disease. Prog Mol Biol Transl Sci. 2020;172:293–323. - PubMed
-
- Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012. Dec 7;151(6):1256–1269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
