Sleep in disorders of consciousness: behavioral and polysomnographic recording
- PMID: 33213463
- PMCID: PMC7678091
- DOI: 10.1186/s12916-020-01812-6
Sleep in disorders of consciousness: behavioral and polysomnographic recording
Abstract
Background: Sleep-wakefulness cycles are an essential diagnostic criterion for disorders of consciousness (DOC), differentiating prolonged DOC from coma. Specific sleep features, like the presence of sleep spindles, are an important marker for the prognosis of recovery from DOC. Based on increasing evidence for a link between sleep and neuronal plasticity, understanding sleep in DOC might facilitate the development of novel methods for rehabilitation. Yet, well-controlled studies of sleep in DOC are lacking. Here, we aimed to quantify, on a reliable evaluation basis, the distribution of behavioral and neurophysiological sleep patterns in DOC over a 24-h period while controlling for environmental factors (by recruiting a group of conscious tetraplegic patients who resided in the same hospital).
Methods: We evaluated the distribution of sleep and wakefulness by means of polysomnography (EEG, EOG, EMG) and video recordings in 32 DOC patients (16 unresponsive wakefulness syndrome [UWS], 16 minimally conscious state [MCS]), and 10 clinical control patients with severe tetraplegia. Three independent raters scored the patients' polysomnographic recordings.
Results: All but one patient (UWS) showed behavioral and electrophysiological signs of sleep. Control and MCS patients spent significantly more time in sleep during the night than during daytime, a pattern that was not evident in UWS. DOC patients (particularly UWS) exhibited less REM sleep than control patients. Forty-four percent of UWS patients and 12% of MCS patients did not have any REM sleep, while all control patients (100%) showed signs of all sleep stages and sleep spindles. Furthermore, no sleep spindles were found in 62% of UWS patients and 21% of MCS patients. In the remaining DOC patients who had spindles, their number and amplitude were significantly lower than in controls.
Conclusions: The distribution of sleep signs in DOC over 24 h differs significantly from the normal sleep-wakefulness pattern. These abnormalities of sleep in DOC are independent of external factors such as severe immobility and hospital environment.
Keywords: EEG; Minimally conscious state; Polysomnography; Sleep; Unresponsive wakefulness; Vegetative state.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


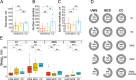
References
-
- Yang X, Song C, Yuan F, Zhao J, Jiang Y, Yang F, et al. Prognostic roles of sleep electroencephalography pattern and circadian rhythm biomarkers in the recovery of consciousness in patients with coma: a prospective cohort study. Sleep Medicine. 2020;69:204–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

