An in vitro bioengineered model of the human arterial neurovascular unit to study neurodegenerative diseases
- PMID: 33213497
- PMCID: PMC7678181
- DOI: 10.1186/s13024-020-00418-z
An in vitro bioengineered model of the human arterial neurovascular unit to study neurodegenerative diseases
Abstract
Introduction: The neurovascular unit (NVU) - the interaction between the neurons and the cerebrovasculature - is increasingly important to interrogate through human-based experimental models. Although advanced models of cerebral capillaries have been developed in the last decade, there is currently no in vitro 3-dimensional (3D) perfusible model of the human cortical arterial NVU.
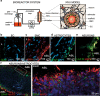
Method: We used a tissue-engineering technique to develop a scaffold-directed, perfusible, 3D human NVU that is cultured in native-like flow conditions that mimics the anatomy and physiology of cortical penetrating arteries.
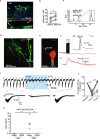
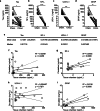
Results: This system, composed of primary human vascular cells (endothelial cells, smooth muscle cells and astrocytes) and induced pluripotent stem cell (iPSC) derived neurons, demonstrates a physiological multilayer organization of the involved cell types. It reproduces key characteristics of cortical neurons and astrocytes and enables formation of a selective and functional endothelial barrier. We provide proof-of-principle data showing that this in vitro human arterial NVU may be suitable to study neurovascular components of neurodegenerative diseases such as Alzheimer's disease (AD), as endogenously produced phosphorylated tau and beta-amyloid accumulate in the model over time. Finally, neuronal and glial fluid biomarkers relevant to neurodegenerative diseases are measurable in our arterial NVU model.
Conclusion: This model is a suitable research tool to investigate arterial NVU functions in healthy and disease states. Further, the design of the platform allows culture under native-like flow conditions for extended periods of time and yields sufficient tissue and media for downstream immunohistochemistry and biochemistry analyses.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

