Human adipose-derived stem cells enriched with VEGF-modified mRNA promote angiogenesis and long-term graft survival in a fat graft transplantation model
- PMID: 33213517
- PMCID: PMC7678328
- DOI: 10.1186/s13287-020-02008-8
Human adipose-derived stem cells enriched with VEGF-modified mRNA promote angiogenesis and long-term graft survival in a fat graft transplantation model
Abstract
Background: Fat grafting, as a standard treatment for numerous soft tissue defects, remains unpredictable and technique-dependent. Human adipose-derived stem cells (hADSCs) are promising candidates for cell-assisted therapy to improve graft survival. As free-living fat requires nutritional and respiratory sources to thrive, insufficient and unstable vascularization still impedes hADSC-assisted therapy. Recently, cytotherapy combined with modified mRNA (modRNA) encoding vascular endothelial growth factor (VEGF) has been applied for the treatment of ischemia-related diseases. Herein, we hypothesized that VEGF modRNA (modVEGF)-engineered hADSCs could robustly enhance fat survival in a fat graft transplantation model.
Methods: hADSCs were acquired from lipoaspiration and transfected with modRNAs. Transfection efficiency and expression kinetics of modRNAs in hADSCs were first evaluated in vitro. Next, we applied an in vivo Matrigel plug assay to assess the viability and angiogenic potential of modVEGF-engineered hADSCs at 1 week post-implantation. Finally, modVEGF-engineered hADSCs were co-transplanted with human fat in a murine model to analyze the survival rate, re-vascularization, proliferation, fibrosis, apoptosis, and necrosis of fat grafts over long-term follow-up.
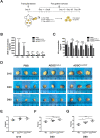
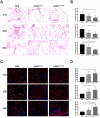
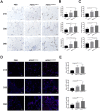
Results: Transfections of modVEGF in hADSCs were highly tolerable as the modVEGF-engineered hADSCs facilitated burst-like protein production of VEGF in both our in vitro and in vivo models. modVEGF-engineered hADSCs induced increased levels of cellular proliferation and proangiogenesis when compared to untreated hADSCs in both ex vivo and in vivo assays. In a fat graft transplantation model, we provided evidence that modVEGF-engineered hADSCs promote the optimal potency to preserve adipocytes, especially in the long-term post-transplantation phase. Detailed histological analysis of fat grafts harvested at 15, 30, and 90 days following in vivo grafting suggested the release of VEGF protein from modVEGF-engineered hADSCs significantly improved neo-angiogenesis, vascular maturity, and cell proliferation. The modVEGF-engineered hADSCs also significantly mitigated the presence of fibrosis, apoptosis, and necrosis of grafts when compared to the control groups. Moreover, modVEGF-engineered hADSCs promoted graft survival and cell differentiation abilities, which also induced an increase in vessel formation and the number of surviving adipocytes after transplantation.
Conclusion: This current study demonstrates the employment of modVEGF-engineered hADSCs as an advanced alternative to the clinical treatment involving soft-tissue reconstruction and rejuvenation.
Keywords: Angiogenesis; Fat transplantation; Graft survival; hADSCs; modVEGF.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures






Similar articles
-
Frozen Fat Grafts Maintain Vascular Endothelial Growth Factor Expression and Mediate Angiogenesis During Adipose-Derived Stem Cell Enrichment for Soft Tissue Augmentation.Ann Plast Surg. 2022 Mar 1;88(1s Suppl 1):S4-S12. doi: 10.1097/SAP.0000000000003075. Ann Plast Surg. 2022. PMID: 35102020
-
Enhanced human adipose-derived stem cells with VEGFA and bFGF mRNA promote stable vascular regeneration and improve cardiac function following myocardial infarction.Clin Transl Med. 2025 Mar;15(3):e70250. doi: 10.1002/ctm2.70250. Clin Transl Med. 2025. PMID: 40008489 Free PMC article.
-
Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells.Plast Reconstr Surg. 2009 Nov;124(5):1437-1446. doi: 10.1097/PRS.0b013e3181babbb6. Plast Reconstr Surg. 2009. PMID: 20009828
-
The Evolving Function of Vasculature and Pro-angiogenic Therapy in Fat Grafting.Cell Transplant. 2024 Jan-Dec;33:9636897241264976. doi: 10.1177/09636897241264976. Cell Transplant. 2024. PMID: 39056562 Free PMC article. Review.
-
Does Secondary Mechanical Manipulation of Lipoaspirate Enhance the Vasculogenic Potential of Fat Grafts? A Systematic Review.Ann Plast Surg. 2024 Sep 1;93(3):389-396. doi: 10.1097/SAP.0000000000004048. Epub 2024 Jul 30. Ann Plast Surg. 2024. PMID: 39150855
Cited by
-
Application of adipose-derived stem cells in treating fibrosis.World J Stem Cells. 2021 Nov 26;13(11):1747-1761. doi: 10.4252/wjsc.v13.i11.1747. World J Stem Cells. 2021. PMID: 34909121 Free PMC article. Review.
-
Hepatocyte growth factor-modified adipose-derived mesenchymal stem cells inhibit human hypertrophic scar fibroblast activation.J Cosmet Dermatol. 2024 Dec;23(12):4268-4276. doi: 10.1111/jocd.16509. Epub 2024 Aug 18. J Cosmet Dermatol. 2024. PMID: 39155606 Free PMC article.
-
Enhancing therapeutic potential: Human adipose-derived mesenchymal stem cells modified with recombinant adeno-associated virus expressing VEGF165 gene for peripheral nerve injury.Kaohsiung J Med Sci. 2024 Sep;40(9):819-829. doi: 10.1002/kjm2.12875. Epub 2024 Aug 5. Kaohsiung J Med Sci. 2024. PMID: 39101328 Free PMC article.
-
Scutellarin protects cortical neurons against neonatal hypoxic-ischemic encephalopathy injury via upregulation of vascular endothelial growth factor.Ibrain. 2022 Jul 9;8(3):353-364. doi: 10.1002/ibra.12052. eCollection 2022 Fall. Ibrain. 2022. PMID: 37786736 Free PMC article.
-
NPTX1 Mediates the Facilitating Effects of Hypoxia-Stimulated Human Adipocytes on Adipose-Derived Stem Cell Activation and Autologous Adipose Graft Survival Rate.Aesthetic Plast Surg. 2024 Oct;48(20):4203-4216. doi: 10.1007/s00266-024-04118-7. Epub 2024 May 24. Aesthetic Plast Surg. 2024. PMID: 38789811
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

