Bioequivalence of levamlodipine besylate tablets in healthy Chinese subjects: a single-dose and two-period crossover randomized study
- PMID: 33213527
- PMCID: PMC7678077
- DOI: 10.1186/s40360-020-00459-6
Bioequivalence of levamlodipine besylate tablets in healthy Chinese subjects: a single-dose and two-period crossover randomized study
Abstract
Background: Levamlodipine, a calcium channel blocker, has been show act as a cardiovascular drug. To compare the pharmacokinetic parameters between levamlodipine (test formulation) at a single dose of 5 mg and amlodipine (reference formulation) at a single dose of 10 mg, the bioequivalence study was carried out.
Methods: A single-dose randomized, open-label, two-period crossover study was designed in healthy Chinese subjects. 48 subjects were divided into fasted and fed groups equally. The subjects randomly received the test or reference formulations at the rate of 1:1. Following a 21-day washout period, the alternative formulations were received. The blood samples were collected at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 24, 36, 48, 72, 96, 120, 144, 168 h later. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was applied to determine the plasma concentrations of levamlodipine. Adverse events were recorded.
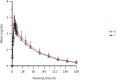
Results: The 90% confidence intervals (CIs) of the ratio of geometric means (GMRs) of Cmax, AUC0-t, and AUC0-∞ under both fasted and fed conditions were within the prespecified bioequivalence limits between 80 ~ 125%. Under fasted conditions, 24 subjects were enrolled and completed the study. The mean Cmax was (2.70 ± 0.49) ng/mL, AUC0-t was (141.32 ± 36.24) ng × h/mL and AUC0-∞ was (157.14 ± 45.65) ng × h/mL after a single dose of 5 mg levamlodipine. The mean Cmax was (2.83 ± 0.52) ng/mL, AUC0-t was (153.62 ± 33.96) ng × h/mL and AUC0-∞ was (173.05 ± 41.78) ng × h/mL after a single dose of 10 mg amlodipine. Under fed conditions, 24 subjects were enrolled and completed the study. The mean Cmax was (2.73 ± 0.55) ng/mL, AUC0-t was (166.93 ± 49.96) ng × h/mL and AUC0-∞ was (190.99 ± 70.89) ng × h/mL after a single dose of 5 mg levamlodipine. The mean Cmax was (2.87 ± 0.81) ng/mL AUC0-t was (165.46 ± 43.58) ng × h/mL and AUC0-∞ was (189.51 ± 64.70) ng × h/mL after a single dose of 10 mg amlodipine. Serious adverse event was not observed.
Conclusion: The trial confirmed that levamlodipine at a single dose of 5 mg and amlodipine at a single dose of 10 mg were bioequivalent under both fasted condition and fed condition.
Trial registration: Cinicaltrials, NCT04411875 . Registered 3 June 2020 - Retrospectively registered.
Keywords: Amlodipine; Bioequivalence; Levamlodipine; Pharmacokinetics.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

References
-
- Lu Y, Yin J, Wu X, Fan Y, Liu F. Comparative effects of 2.5mg levamlodipine and 5mg amlodipine on vascular endothelial function and atherosclerosis. Pak J Pharm Sci. 2019;32(5(Special)):2433–2436. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

