Regulation of Cardiovascular Therapies During the COVID-19 Public Health Emergency
- PMID: 33213730
- PMCID: PMC7669239
- DOI: 10.1016/j.jacc.2020.09.594
Regulation of Cardiovascular Therapies During the COVID-19 Public Health Emergency
Keywords: COVID-19; clinical trials; coronavirus; regulation.
Conflict of interest statement
Author Relationship With Industry Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541); has received research support from Amgen; has served on Advisory Boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa; and has participated on clinical endpoint committees for studies sponsored by Galmed, Novartis, and the National Institutes of Health. Dr. Butler has served as a consultant to Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, BerlinCures, Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave Limited, and Vifor. Dr. Krumholz has received expenses and/or personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing; is an owner of Refactor Health and HugoHealth; and has had grants and/or contracts from the Centers for Medicare & Medicaid Services, Medtronic, the U.S. Food and Drug Administration, Johnson & Johnson, and the Shenzhen Center for Health Information. Dr. Bhatt has served on the Advisory Boards of Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, and Regado Biosciences; has served on the boards of directors of the Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; has served as chair of the American Heart Association Quality Oversight Committee; has served on data monitoring committees for the Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards Lifesciences), Contego Medical (chair of the PERFORMANCE 2 trial), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi-Sankyo), and Population Health Research Institute; has received honoraria from the American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org; and vice chair of the ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor; associate editor), K2P (co-chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (CME steering committees); has other relationships with Clinical Cardiology (deputy editor), NCDR-ACTION Registry Steering Committee (chair), and VA CART Research and Publications Committee (chair); has received research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, and The Medicines Company; has received royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); has been a site coinvestigator for Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; has been a trustee for the American College of Cardiology; and has performed unfunded research for FlowCo, Merck, Novo Nordisk, and Takeda. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
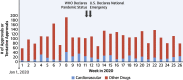
References
-
- U.S. Food and Drug Administration . 2020. Novel Drug Approvals for 2020.https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and... Available at:
-
- U.S. Food and Drug Administration . 2020. 2020 Device Approvals.https://www.fda.gov/medical-devices/recently-approved-devices/2020-devic... Available at:
-
- U.S. Food and Drug Administration . 2020. Drugs@FDA: FDA-Approved Drugs.https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=reportsS... Available at:
-
- U.S. Food and Drug Administration Drug Shortages. https://www.accessdata.fda.gov/scripts/drugshortages/ Available at: Accessed on July 14, 2020.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

