Neutrophils interact with cholangiocytes to cause cholestatic changes in alcoholic hepatitis
- PMID: 33214166
- PMCID: PMC7906004
- DOI: 10.1136/gutjnl-2020-322540
Neutrophils interact with cholangiocytes to cause cholestatic changes in alcoholic hepatitis
Abstract
Background & objectives: Alcoholic hepatitis (AH) is a common but life-threatening disease with limited treatment options. It is thought to result from hepatocellular damage, but the presence of cholestasis worsens prognosis, so we examined whether bile ducts participate in the pathogenesis of this disease.
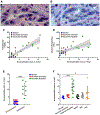
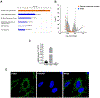
Design: Cholangiocytes derived from human bile ducts were co-cultured with neutrophils from patients with AH or controls. Loss of type 3 inositol 1,4,5-trisphosphate receptor (ITPR3), an apical intracellular calcium channel necessary for cholangiocyte secretion, was used to reflect cholestatic changes. Neutrophils in contact with bile ducts were quantified in liver biopsies from patients with AH and controls and correlated with clinical and pathological findings.
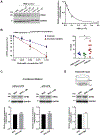
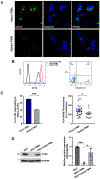
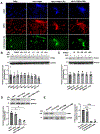
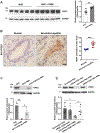
Results: Liver biopsies from patients with AH revealed neutrophils in contact with bile ducts, which correlated with biochemical and histological parameters of cholestasis. Cholangiocytes co-cultured with neutrophils lost ITPR3, and neutrophils from patients with AH were more potent than control neutrophils. Biochemical and histological findings were recapitulated in an AH animal model. Loss of ITPR3 was attenuated by neutrophils in which surface membrane proteins were removed. RNA-seq analysis implicated integrin β1 (ITGB1) in neutrophil-cholangiocyte interactions and interference with ITGB1 on cholangiocytes blocked the ability of neutrophils to reduce cholangiocyte ITPR3 expression. Cell adhesion molecules on neutrophils interacted with ITGB1 to trigger RAC1-induced JNK activation, causing a c-Jun-mediated decrease in ITPR3 in cholangiocytes.
Conclusions: Neutrophils bind to ITGB1 on cholangiocytes to contribute to cholestasis in AH. This previously unrecognised role for cholangiocytes in this disease alters our understanding of its pathogenesis and identifies new therapeutic targets.
Keywords: alcoholic liver disease; bilirubin; chemokines; cholestasis.
© Author(s) (or their employer(s)) 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
