COVID-19-Associated Glomerular Disease
- PMID: 33214201
- PMCID: PMC7894674
- DOI: 10.1681/ASN.2020060804
COVID-19-Associated Glomerular Disease
Abstract
Background: Studies have documented AKI with high-grade proteinuria in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In some patients, biopsies have revealed collapsing glomerulopathy, a distinct form of glomerular injury that has been associated with other viruses, including HIV. Previous patient reports have described patients of African ancestry who developed nephrotic-range proteinuria and AKI early in the course of disease.
Methods: In this patient series, we identified six patients with coronavirus disease 2019 (COVID-19), AKI, and nephrotic-range proteinuria. COVID-19 was diagnosed by a positive nasopharyngeal swab RT-PCR for SARS-CoV-2 infection. We examined biopsy specimens from one transplanted kidney and five native kidneys. Three of the six patients underwent genetic analysis of APOL1, the gene encoding the APOL1 protein, from DNA extracted from peripheral blood. In addition, we purified genomic DNA from paraffin-embedded tissue and performed APOL1 genotype analysis of one of the native biopsies and the donor kidney graft.
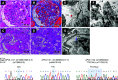
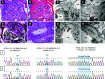
Results: All six patients were of recent African ancestry. They developed COVID-19-associated AKI with podocytopathy, collapsing glomerulopathy, or both. Patients exhibited generally mild respiratory symptoms, and no patient required ventilator support. Genetic testing performed in three patients confirmed high-risk APOL1 genotypes. One APOL1 high-risk patient developed collapsing glomerulopathy in the engrafted kidney, which was transplanted from a donor who carried a low-risk APOL1 genotype; this contradicts current models of APOL1-mediated kidney injury, and suggests that intrinsic renal expression of APOL1 may not be the driver of nephrotoxicity and specifically, of podocyte injury.
Conclusions: Glomerular disease presenting as proteinuria with or without AKI is an important presentation of COVID-19 infection and may be associated with a high-risk APOL1 genotype.
Keywords: APOL1; COVID-19; collapsing glomerulopathy.
Copyright © 2021 by the American Society of Nephrology.
Figures

Comment in
-
COVID-19 and APOL1: Understanding Disease Mechanisms through Clinical Observation.J Am Soc Nephrol. 2021 Jan;32(1):1-2. doi: 10.1681/ASN.2020111629. Epub 2020 Dec 7. J Am Soc Nephrol. 2021. PMID: 33288631 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

