Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis
- PMID: 33214209
- PMCID: PMC8156151
- DOI: 10.1183/13993003.02559-2020
Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis
Erratum in
-
"Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis." Nikhil Hirani, Alison C. MacKinnon, Lisa Nicol, et al. Eur Respir J 2021; 57: 2002559.Eur Respir J. 2022 Apr 14;59(4):2052559. doi: 10.1183/13993003.52559-2020. Print 2022 Apr. Eur Respir J. 2022. PMID: 35422427 Free PMC article. No abstract available.
Abstract
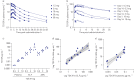
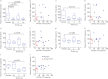
Galectin (Gal)-3 is a profibrotic β-galactoside-binding lectin that plays a key role in the pathogenesis of idiopathic pulmonary fibrosis (IPF) and IPF exacerbations. TD139 is a novel and potent small-molecule inhibitor of Gal-3.A randomised, double-blind, multicentre, placebo-controlled, phase 1/2a study was conducted to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of inhaled TD139 in 36 healthy subjects and 24 patients with IPF. Six dose cohorts of six healthy subjects were evaluated (4:2 TD139:placebo ratio) with single doses of TD139 (0.15-50 mg) and three dose cohorts of eight patients with IPF (5:3 TD139:placebo ratio) with once-daily doses of TD139 (0.3-10 mg) for 14 days.Inhaled TD139 was well tolerated with no significant treatment-related side-effects. TD139 was rapidly absorbed, with mean time taken to reach maximum plasma concentration (C max) values ranging from 0.6 to 3 h and a plasma half-life (T 1/2) of 8 h. The concentration of TD139 in the lung was >567-fold higher than in the blood, with systemic exposure predicting exposure in the target compartment. Gal-3 expression on alveolar macrophages was reduced in the 3 and 10 mg dose groups compared with placebo, with a concentration-dependent inhibition demonstrated. Inhibition of Gal-3 expression in the lung was associated with reductions in plasma biomarkers centrally relevant to IPF pathobiology (platelet-derived growth factor-BB, plasminogen activator inhibitor-1, Gal-3, CCL18 and YKL-40).TD139 is safe and well tolerated in healthy subjects and IPF patients. It was shown to suppress Gal-3 expression on bronchoalveolar lavage macrophages and, in a concerted fashion, decrease plasma biomarkers associated with IPF progression.
Trial registration: ClinicalTrials.gov NCT02257177.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: N. Hirani reports grants from Galecto Biotech, during the conduct of the study. Conflict of interest: A.C. MacKinnon reports personal fees from Galecto Biotech, outside the submitted work; and has a patent CA2,794,066 issued, a patent US13/832,672 issued and a patent WO/2014/067986 pending (all patents are fully owned by Galecto Biotech). Conflict of interest: L. Nicol reports grants from Galecto Biotech, during the conduct of the study; personal fees for lectures from Boehringer Ingelheim, outside the submitted work. Conflict of interest: P. Ford reports personal fees and nonfinancial support from Galecto, during the conduct of the study; and has a patent TD139 issued. Conflict of interest: H. Schambye reports personal fees from Galecto Inc, outside the submitted work; and has a patent WO/2016/180483 pending (fully owned by Galecto Biotech). Conflict of interest: A. Pedersen reports personal fees from Galecto Biotech, outside the submitted work. Conflict of interest: U.J. Nilsson has a patent CA2,794,066 issued, a patent US13/832,672 issued, a patent WO/2014/067986 pending, a patent WO/2005/113569 pending and a patent WO/2009/139719 pending (all patents are fully owned by Galecto Biotech). Conflict of interest: H. Leffler has a patent CA2,794,066 issued, a patent US13/832,672 issued, a patent WO/2014/067986 pending and a patent WO/2005/113569 pending (all patents are fully owned by Galecto Biotech). Conflict of interest: T. Sethi reports personal fees from Galecto Biotech, outside the submitted work; and has a patent CA2,794,066 issued, a patent US13/832,672 issued and a patent WO/2014/067986 pending (patents are fully owned by Galecto Biotech). Conflict of interest: S. Tantawi reports personal fees from Galecto Biotech, outside the submitted work. Conflict of interest: L. Gravelle reports personal fees from Galecto Biotech, outside the submitted work; and has a patent WO/2017/103109 pending (fully owned by Galecto Biotech). Conflict of interest: R.J. Slack reports personal fees from Galecto Biotech, outside the submitted work. Conflict of interest: R. Mills has nothing to disclose. Conflict of interest: U. Karmakar has nothing to disclose. Conflict of interest: D. Humphries has nothing to disclose. Conflict of interest: F. Zetterberg reports personal fees from Galecto Biotech, outside the submitted work. Conflict of interest: L. Keeling has nothing to disclose. Conflict of interest: L. Paul has nothing to disclose. Conflict of interest: P.L. Molyneaux has, via his institution, received industry-academic funding from AstraZeneca and has received speaker and consultancy fees from Boehringer Ingelheim and Hoffman-La Roche, outside the submitted work. Conflict of interest: F. Li has nothing to disclose. Conflict of interest: W. Funston has nothing to disclose. Conflict of interest: I.A. Forrest reports personal fees for consultancy and meeting attendance from Boehringer Ingelheim, personal fees for lectures and meeting attendance from Roche Ltd, outside the submitted work. Conflict of interest: A.J. Simpson has nothing to disclose. Conflict of interest: M.A. Gibbons has nothing to disclose. Conflict of interest: T.M. Maher has, via his institution, received industry-academic funding from AstraZeneca and GlaxoSmithKline R&D, and has received consultancy or speaker fees from AstraZeneca, Bayer, Blade Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Galapagos, GlaxoSmithKline R&D, Indalo, Novartis, Pliant, Respivant, Roche and Samumed.
Figures


Comment in
-
Repeat bronchoalveolar lavage in idiopathic pulmonary fibrosis: proceed with caution?Eur Respir J. 2021 May 27;57(5):2100691. doi: 10.1183/13993003.00691-2021. Print 2021 May. Eur Respir J. 2021. PMID: 34045285 No abstract available.
-
New Frontiers in Therapeutics for Interstitial Lung Diseases.Am J Respir Crit Care Med. 2023 Apr 15;207(8):1089-1091. doi: 10.1164/rccm.202206-1035RR. Am J Respir Crit Care Med. 2023. PMID: 36735934 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
