Murine Cytomegalovirus-induced Complement-fixing Antibodies Deposit in Murine Renal Allografts During Acute Rejection
- PMID: 33214535
- PMCID: PMC8128940
- DOI: 10.1097/TP.0000000000003548
Murine Cytomegalovirus-induced Complement-fixing Antibodies Deposit in Murine Renal Allografts During Acute Rejection
Abstract
Background: Human cytomegalovirus (CMV) infection is associated with renal allograft dysfunction and loss, particularly in combination with acute rejection. Emerging literature suggests that non-HLA antibodies may contribute to antibody-mediated rejection, but pathogen-induced antibodies have not been investigated in this context. This study examines the presence of CMV-induced antibodies in murine CMV (MCMV)-infected renal allografts during acute rejection.
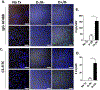
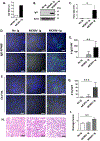
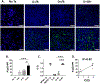
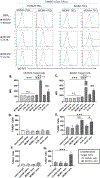
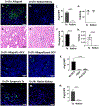
Methods: Intragraft immunoglobulin G (IgG) and complement C3 immunostaining were compared among allogeneic MCMV D-/R-, D+/R-, and D+/R+ renal transplants. Intragraft antibody deposition was examined in B cell-deficient recipients treated with MCMV immune sera. Antibody binding and complement-dependent cytotoxicity (CDC) of D-/R- and D+/R+ sera against infected renal tubular epithelial cells (TECs) were measured in vitro. IgG immunostaining was performed in D+/R+ allografts and native kidneys and in D+/R- allografts treated with ganciclovir to inhibit viral replication.
Results: D+/R- and D+/R+ transplants had more abundant IgG and C3 deposition compared with D-/R- recipients. Greater IgG deposition was associated with more severe allograft injury in B cell-deficient recipients treated with MCMV immune sera compared with nonimmune sera. D+/R+ sera induced greater CDC of infected TECs compared with D-/R- sera. Native kidneys had lower IgG deposition compared with allografts, despite similar organ viral loads. Ganciclovir-treated allografts had reduced IgG deposition compared with untreated allografts.
Conclusions: In this murine model, complement-fixing antibodies can deposit into MCMV-infected renal allografts, are associated with allograft damage, and can induce CDC of MCMV-infected renal TECs. The allogeneic response and viral replication may also contribute to intragraft antibody deposition.
Copyright © 2020 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Comment in
-
Cytomegalovirus: Another Source of Antibody-mediated Graft Injury?Transplantation. 2021 Aug 1;105(8):1653-1654. doi: 10.1097/TP.0000000000003549. Transplantation. 2021. PMID: 33214536 No abstract available.
References
-
- Freeman RB Jr. The ‘Indirect’ effects of cytomegalovirus infection. American Journal of Transplantation. 2009;9(11):2453–2458. - PubMed
-
- Tong CYW, Bakran A, Peiris JSM, et al. The association of viral infection and chronic allograft nephropathy with graft dysfunction after renal transplantation. Transplantation. 2002;74:576–578. - PubMed
-
- Legendre C, Pascual M. Improving outcomes for solid organ transplant recipients at risk from cytomegalovirus infection: late onset disease and indirect consequences. Clinical Infectious Diseases. 2008;46(5):732–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

