Loss of the spectraplakin gene Short stop induces a DNA damage response in Drosophila epithelia
- PMID: 33214581
- PMCID: PMC7677407
- DOI: 10.1038/s41598-020-77159-y
Loss of the spectraplakin gene Short stop induces a DNA damage response in Drosophila epithelia
Abstract
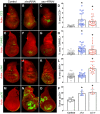
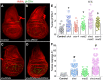
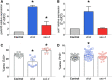
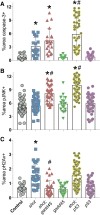
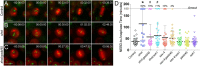
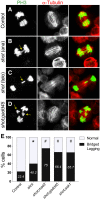
Epithelia are an eminent tissue type and a common driver of tumorigenesis, requiring continual precision in cell division to maintain tissue structure and genome integrity. Mitotic defects often trigger apoptosis, impairing cell viability as a tradeoff for tumor suppression. Identifying conditions that lead to cell death and understanding the mechanisms behind this response are therefore of considerable importance. Here we investigated how epithelia of the Drosophila wing disc respond to loss of Short stop (Shot), a cytoskeletal crosslinking spectraplakin protein that we previously found to control mitotic spindle assembly and chromosome dynamics. In contrast to other known spindle-regulating genes, Shot knockdown induces apoptosis in the absence of Jun kinase (JNK) activation, but instead leads to elevated levels of active p38 kinase. Shot loss leads to double-strand break (DSB) DNA damage, and the apoptotic response is exacerbated by concomitant loss of p53. DSB accumulation is increased by suppression of the spindle assembly checkpoint, suggesting this effect results from chromosome damage during error-prone mitoses. Consistent with DSB induction, we found that the DNA damage and stress response genes, Growth arrest and DNA damage (GADD45) and Apoptosis signal-regulating kinase 1 (Ask1), are transcriptionally upregulated as part of the shot-induced apoptotic response. Finally, co-depletion of Shot and GADD45 induced significantly higher rates of chromosome segregation errors in cultured cells and suppressed shot-induced mitotic arrest. Our results demonstrate that epithelia are capable of mounting molecularly distinct responses to loss of different spindle-associated genes and underscore the importance of proper cytoskeletal organization in tissue homeostasis.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

