A serological assay to detect SARS-CoV-2 antibodies in at-home collected finger-prick dried blood spots
- PMID: 33214612
- PMCID: PMC7678827
- DOI: 10.1038/s41598-020-76913-6
A serological assay to detect SARS-CoV-2 antibodies in at-home collected finger-prick dried blood spots
Abstract
Accurate surveillance of coronavirus disease 2019 (COVID-19) incidence requires large-scale testing of the population. Current testing methods require in-person collection of biospecimens by a healthcare worker, limiting access of individuals who do not have access to testing facilities while placing both patients and healthcare workers at risk of exposure to infection. We report the development and validation of a at-home finger-prick dried blood spot collection kit and an analysis method. We demonstrated 100% sensitivity and specificity using at-home collected specimens across the US. Such methods may facilitate the conduct of unbiased serosurveys within hard to reach populations and help reduce the sample collection burden of serological testing on both health care systems and individuals alike.
Conflict of interest statement
D.G.K., K.D., N.F., D.S., P.V.R. and C.T.T are employees of Enable Biosciences. K.D., P.V.R., N.F., D.S. and C.T.T are shareholders of Enable Biosciences. P.V.R and C.T.T are inventors of the ADAP patent licensed from University of California, Berkeley to Enable Biosciences. The ADAP assay used in this assay is a product in development. This does not alter our adherence to Scientific Reports policies on sharing data and materials.
Figures
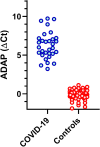
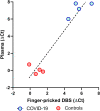
Update of
-
A serological assay to detect SARS-CoV-2 antibodies in at-home collected finger-prick dried blood spots.medRxiv [Preprint]. 2020 Jun 3:2020.05.29.20116004. doi: 10.1101/2020.05.29.20116004. medRxiv. 2020. Update in: Sci Rep. 2020 Nov 19;10(1):20188. doi: 10.1038/s41598-020-76913-6. PMID: 32511529 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

