Serum trace metal association with response to erythropoiesis stimulating agents in incident and prevalent hemodialysis patients
- PMID: 33214633
- PMCID: PMC7677396
- DOI: 10.1038/s41598-020-77311-8
Serum trace metal association with response to erythropoiesis stimulating agents in incident and prevalent hemodialysis patients
Abstract
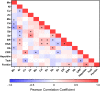
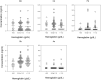
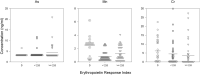
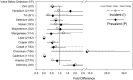
Alterations in hemodialysis patients' serum trace metals have been documented. Early studies addressing associations levels of serum trace metals with erythropoietic responses and/or hematocrit generated mixed results. These studies were conducted prior to current approaches for erythropoiesis stimulating agent (ESA) drug dosing guidelines or without consideration of inflammation markers (e.g. hepcidin) important for regulation of iron availability. This study sought to determine if the serum trace metal concentrations of incident or chronic hemodialysis patients associated with the observed ESA response variability and with consideration to ESA dose response, hepcidin, and high sensitivity C-reactive protein levels. Inductively-coupled plasma-mass spectrometry was used to measure 14 serum trace metals in 29 incident and 79 prevalent dialysis patients recruited prospectively. We compared these data to three measures of ESA dose response, sex, and dialysis incidence versus dialysis prevalence. Hemoglobin was negatively associated with ESA dose and cadmium while positively associated with antimony, arsenic and lead. ESA dose was negatively associated with achieved hemoglobin and vanadium while positively associated with arsenic. ESA response was positively associated with arsenic. Vanadium, nickel, cadmium, and tin were increased in prevalent patients. Manganese was increased in incident patients. Vanadium, nickel, and arsenic increased with time on dialysis while manganese decreased. Changes in vanadium and manganese were largest and appeared to have some effect on anemia. Incident and prevalent patients' chromium and antimony levels exceeded established accepted upper limits of normal.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES030283/ES/NIEHS NIH HHS/United States
- R01 DK091584/DK/NIDDK NIH HHS/United States
- R01DK091584/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)/International
- K01GM109320/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)/International
- U24DK097193/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)/International
LinkOut - more resources
Full Text Sources
Medical
Research Materials

