Purification and biochemical characterization of Hel a 6, a cross-reactive pectate lyase allergen from Sunflower (Helianthus annuus L.) pollen
- PMID: 33214682
- PMCID: PMC7677321
- DOI: 10.1038/s41598-020-77247-z
Purification and biochemical characterization of Hel a 6, a cross-reactive pectate lyase allergen from Sunflower (Helianthus annuus L.) pollen
Expression of concern in
-
Editorial Expression of Concern: Purification and biochemical characterization of Hel a 6, a cross-reactive pectate lyase allergen from Sunflower (Helianthus annuus L.) pollen.Sci Rep. 2024 Oct 1;14(1):22820. doi: 10.1038/s41598-024-74241-7. Sci Rep. 2024. PMID: 39354202 Free PMC article. No abstract available.
Abstract
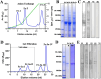
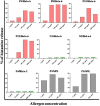
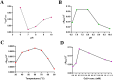
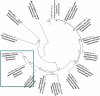
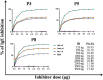
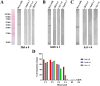
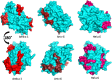
Sunflower pollen was reported to contain respiratory allergens responsible for occupational allergy and pollinosis. The present study describes the comprehensive characterization of a major sunflower allergen Hel a 6. Natural Hel a 6 was purified from sunflower pollen by anion exchange and gel filtration chromatography. Hel a 6 reacted with IgE-antibodies from 57% of 39 sunflower-sensitized patient sera suggesting it to be a major allergen. The patients were of Indian origin and suffering from pollinosis and allergic rhinitis. Hel a 6 exhibited allergenic activity by stimulating mediator release from basophils. Monomeric Hel a 6 displayed pectate lyase activity. The effect of various physicochemical parameters such as temperature, pH, and calcium ion on the functional activity of Hel a 6 revealed a stable nature of the protein. Hel a 6 was folded, and its melting curve showed reversible denaturation in which it refolded back to its native conformation from a denatured state. Hel a 6 displayed a high degree of sequence conservation with the pectate lyase allergens from related taxonomic families such as Amb a 1 (67%) and Art v 6 (57%). The IgE-cross reactivity was observed between Hel a 6 and its ragweed and mugwort homologs. The cross-reactivity was further substantiated by the mediator release when Hel a 6-sensitized effector cells were cross-stimulated with Art v 6 and Amb a 1. Several putative B cell epitopes were predicted and mapped on these 3 allergens. Two antigenic regions were found to be commonly shared by these 3 allergens, which could be crucial for cross-reactivity. In conclusion, Hel a 6 serves as a candidate molecule for diagnosis and immunotherapy for weed allergy.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

