Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19
- PMID: 33214719
- PMCID: PMC7675406
- DOI: 10.1038/s41577-020-00470-2
Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19). Understanding of the fundamental processes underlying the versatile clinical manifestations of COVID-19 is incomplete without comprehension of how different immune cells are recruited to various compartments of virus-infected lungs, and how this recruitment differs among individuals with different levels of disease severity. As in other respiratory infections, leukocyte recruitment to the respiratory system in people with COVID-19 is orchestrated by specific leukocyte trafficking molecules, and when uncontrolled and excessive it results in various pathological complications, both in the lungs and in other organs. In the absence of experimental data from physiologically relevant animal models, our knowledge of the trafficking signals displayed by distinct vascular beds and epithelial cell layers in response to infection by SARS-CoV-2 is still incomplete. However, SARS-CoV-2 and influenza virus elicit partially conserved inflammatory responses in the different respiratory epithelial cells encountered early in infection and may trigger partially overlapping combinations of trafficking signals in nearby blood vessels. Here, we review the molecular signals orchestrating leukocyte trafficking to airway and lung compartments during primary pneumotropic influenza virus infections and discuss potential similarities to distinct courses of primary SARS-CoV-2 infections. We also discuss how an imbalance in vascular activation by leukocytes outside the airways and lungs may contribute to extrapulmonary inflammatory complications in subsets of patients with COVID-19. These multiple molecular pathways are potential targets for therapeutic interventions in patients with severe COVID-19.
Conflict of interest statement
The authors declare no competing interests.
Figures
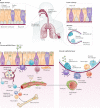
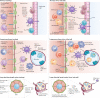

References
-
- Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 2008;8:142–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

