Knowledge, Perceptions, and Uptake of the Human Papillomavirus Vaccine in a Sample of US High School Adolescents
- PMID: 33214780
- PMCID: PMC7671009
- DOI: 10.5863/1551-6776-25.8.697
Knowledge, Perceptions, and Uptake of the Human Papillomavirus Vaccine in a Sample of US High School Adolescents
Abstract
Objective: To assess high school students' knowledge and perceptions of human papillomavirus (HPV) and HPV vaccines and evaluate high school students' self-reported uptake of the HPV vaccine.
Methods: This was an observational, descriptive study using a 44-question survey. Participants were ninth grade students in a Colorado public school district. The survey was administered as part of a health education course.
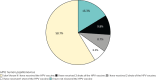
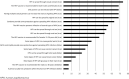
Results: Ninety-two surveys were included in the analysis. Demographic characteristics included 64/92 (69.6%) male and 55/92 (59.8%) Hispanic/Latino students. There was no difference between males and females regarding self-reported vaccination status. Non-Hispanic students were 71.6% less likely to have received the HPV vaccine than Hispanic students (OR 0.284; 95% CI, 0.088-0.920; p = 0.036). The average score on the knowledge section was 42.7% with a standard deviation of 22.6%. When assessing students' perceptions, 71/92 (77.2%) disagreed or strongly disagreed that they felt at risk for getting an HPV infection. There was no significant difference between males and females regarding awareness of the HPV vaccine (p = 0.14). More than half of students (58.7%) did not know if they had received the HPV vaccine.
Conclusion: HPV vaccine awareness was low and many students did not know if they had received the HPV vaccine. Ninth grade students did not have accurate knowledge of HPV and HPV vaccines and this study presents opportunities for increased education.
Keywords: Hispanic Americans; adolescent; health education; health surveys; papillomavirus infections; papillomavirus vaccines; vaccination.
Copyright Pediatric Pharmacy Association. All rights reserved. For permissions, email: mhelms@pediatricpharmacy.org 2020.
Conflict of interest statement
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
Similar articles
-
Human Papillomavirus awareness, knowledge and vaccine acceptance: a survey among 18-25 year old male and female vocational school students in Berlin, Germany.Eur J Public Health. 2012 Dec;22(6):808-13. doi: 10.1093/eurpub/ckr188. Epub 2011 Dec 23. Eur J Public Health. 2012. PMID: 22199161
-
Knowledge, attitudes and factors associated with acceptability of human papillomavirus vaccination among undergraduate medical, dental and nursing students in South India.Hum Vaccin Immunother. 2019;15(7-8):1656-1665. doi: 10.1080/21645515.2019.1565260. Epub 2019 Feb 20. Hum Vaccin Immunother. 2019. PMID: 30648913 Free PMC article.
-
Sex differences among college students in awareness of the human papillomavirus vaccine and vaccine options.J Am Coll Health. 2015;63(2):144-7. doi: 10.1080/07448481.2014.975720. Epub 2015 Jan 20. J Am Coll Health. 2015. PMID: 25337670
-
Missed opportunities for catch-up human papillomavirus vaccination among university undergraduates: Identifying health decision-making behaviors and uptake barriers.Vaccine. 2018 Jan 4;36(2):331-341. doi: 10.1016/j.vaccine.2017.07.041. Epub 2017 Jul 26. Vaccine. 2018. PMID: 28755837
-
Awareness of and willingness to be vaccinated by human papillomavirus vaccine among junior middle school students in Jinan, China.Hum Vaccin Immunother. 2018 Feb 1;14(2):404-411. doi: 10.1080/21645515.2017.1393132. Epub 2017 Dec 1. Hum Vaccin Immunother. 2018. PMID: 29048994 Free PMC article.
Cited by
-
Factors Associated With Adolescents' Human Papillomavirus Vaccination Intention: A Cross-Sectional Survey.Nurs Open. 2024 Dec;11(12):e70110. doi: 10.1002/nop2.70110. Nurs Open. 2024. PMID: 39647922 Free PMC article.
References
-
- Centers for Disease Control and Prevention Atlanta, GA: CDC; 2018. Sexually transmitted disease surveillance 2018. Accessed November 25, 2019. https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf.
-
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–1408. - PubMed
-
- Gardasil 9 (human papillomavirus 9valent vaccine recombinant) [package insert] Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2019. Accessed November 25, 2019. www.merck.com/product/usa/pi_circulars/g/gardasil_9/gardasil_9_pi.pdf.
LinkOut - more resources
Full Text Sources